

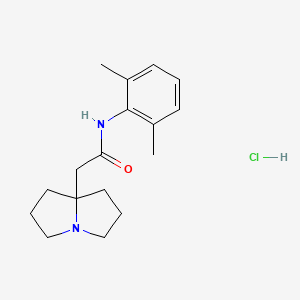

1. 1h-pyrrolizine-7a(5h)-acetamide, N-(2,6-dimethylphenyl)tetrahydro-, Hydrochloride (1:1)
2. 1h-pyrrolizine-7a(5h)-acetamide, N-(2,6-dimethylphenyl)tetrahydro-, Hydrochloride Hemihydrate
3. N-(2,6-dimethylphenyl)-8-pyrrolizidineacetamide Hydrochloride
4. Pilsicainide
5. Pilsicainide Hydrochloride Hemihydrate
6. Pilsicainide Hydrochloride Hydrate
7. Sun 1165
8. Sun-1165
9. Tetrahydro-1h-pyrrolizine-7a(5h)-aceto-2',6'-xylidide
1. 88069-49-2
2. Pilsicainide Hcl
3. N-(2,6-dimethylphenyl)-2-(hexahydro-1h-pyrrolizin-7a-yl)acetamide Hydrochloride
4. Sunrythm
5. Pilsicainide (hydrochloride)
6. Sun 1165
7. Pilsicainide Hydrochoride
8. Sun 1165; Sunrythm
9. 88069-49-2 (hcl)
10. 03c8i9296v
11. Sun-1165
12. N-(2,6-dimethylphenyl)-2-(tetrahydro-1h-pyrrolizin-7a(5h)-yl)acetamide Hydrochloride
13. N-(2,6-dimethylphenyl)-2-(1,2,3,5,6,7-hexahydropyrrolizin-8-yl)acetamide;hydrochloride
14. Unii-03c8i9296v
15. Pilsicainide Hydrochloride [jan]
16. N-(2,6-dimethylphenyl)-1h-pyrrolizine-8-acetamide Hydrochloride
17. N-(2,6-dimethylphenyl)-8-pyrrolizidineacetamide Monohydrochloride
18. 1h-pyrrolizine-8-acetamide, Hexahydro-n-(2,6-dimethylphenyl)-, Hydrochloride
19. N-(2,6-dimethylphenyl)-2-(1,2,3,5,6,7-hexahydropyrrolizin-8-yl)acetamide Hydrochloride
20. Schembl483632
21. Chembl4303511
22. Dtxsid7057860
23. Amy8863
24. Bcp09636
25. Mfcd00903769
26. Akos025287418
27. Ds-9555
28. Du-6552
29. 1h-pyrrolizine-7a(5h)-acetamide, N-(2,6-dimethylphenyl)tetrahydro-, Monohydrochloride
30. Pilsicainide Hydrochloride [who-dd]
31. Hy-101245
32. Pilsicainide Hydrochloride, >=98% (hplc)
33. Cs-0021031
34. Ft-0630955
35. P2634
36. 69p492
37. A16362
38. C76901
39. A842458
40. Q27247552
41. 1h-pyrrolizine-7a(5h)-acetamide, N-(2,6-dimethylphenyl)tetrahydro-, Hydrochloride (1:1)
42. N-(2,6-dimethylphenyl)-2-(1,2,3,5,6,7-hexahydropyrrolizin-8-yl)acetamide,hydrochloride
43. N-(2,6-dimethylphenyl)-2-(1,2,3,5,6,7-hexahydropyrrolizin-8-yl)ethanamide Hydrochloride
44. N-(2,6-dimethylphenyl)-2-(hexahydro-1h-pyrrolizin-7a-yl)acetamide Hcl
45. N-(2,6-dimethylphenyl)-2-(hexahydro-1h-pyrrolizin-7a-yl)acetamidehydrochloride
| Molecular Weight | 308.8 g/mol |
|---|---|
| Molecular Formula | C17H25ClN2O |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 3 |
| Exact Mass | 308.1655411 g/mol |
| Monoisotopic Mass | 308.1655411 g/mol |
| Topological Polar Surface Area | 32.3 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 348 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Anti-Arrhythmia Agents
Agents used for the treatment or prevention of cardiac arrhythmias. They may affect the polarization-repolarization phase of the action potential, its excitability or refractoriness, or impulse conduction or membrane responsiveness within cardiac fibers. Anti-arrhythmia agents are often classed into four main groups according to their mechanism of action: sodium channel blockade, beta-adrenergic blockade, repolarization prolongation, or calcium channel blockade. (See all compounds classified as Anti-Arrhythmia Agents.)
Sodium Channel Blockers
A class of drugs that act by inhibition of sodium influx through cell membranes. Blockade of sodium channels slows the rate and amplitude of initial rapid depolarization, reduces cell excitability, and reduces conduction velocity. (See all compounds classified as Sodium Channel Blockers.)