





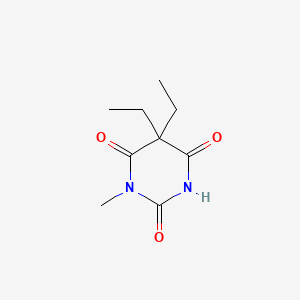

1. Endiemal
2. Metharbitone
3. Methobarbitone
1. Metharbitone
2. Methylbarbital
3. Gemonil
4. Metabarbital
5. N-methylbarbital
6. Endiemalum
7. Metharbutal
8. 50-11-3
9. 1-methylbarbital
10. Endiemal
11. Gemonit
12. 5,5-diethyl-1-methylbarbituric Acid
13. 5,5-diethyl-1-methyl-1,3-diazinane-2,4,6-trione
14. Sch 412
15. An 23
16. Metharbital Ciii
17. Nsc 27156
18. 2,4,6(1h,3h,5h)-pyrimidinetrione, 5,5-diethyl-1-methyl-
19. Barbituric Acid, 5,5-diethyl-1-methyl-
20. Methabarbital
21. Nsc-27156
22. 5,5-diethyl-1-methylpyrimidine-2,4,6(1h,3h,5h)-trione
23. Methabarbitone
24. Metarbitale [dcit]
25. 02os7k758t
26. Metharbital Ciii (200 Mg)
27. Metarbital [inn-spanish]
28. Ncgc00181148-01
29. Metarbitale
30. Metharbitalum
31. Metarbital
32. Metharbitalum [inn-latin]
33. Gemonil (tn)
34. Metharbital (jan/inn)
35. Einecs 200-011-2
36. Brn 0184688
37. Unii-02os7k758t
38. 5,5-diethyl-1-methyl-2,4,6(1h,3h,5h)-pyrimidinetrione
39. Metharbital [usp:inn:ban:jan]
40. Metharbital [mi]
41. Metharbital [inn]
42. Metharbital [jan]
43. Dsstox_cid_3280
44. Chembl450
45. Metharbital [vandf]
46. Dsstox_rid_76955
47. Metharbital [mart.]
48. Dsstox_gsid_23280
49. Schembl78867
50. Metharbital [who-dd]
51. 5-24-09-00144 (beilstein Handbook Reference)
52. Gtpl7230
53. Dtxsid6023280
54. Schembl22556220
55. Chebi:31827
56. Metharbital [orange Book]
57. Metharbital 1.0 Mg/ml In Methanol
58. Nsc27156
59. Zinc5508997
60. Tox21_112752
61. Barbituric Acid,5-diethyl-1-methyl-
62. 1-methyl-5,5-diethyl Barbituric Acid
63. Akos006239548
64. Wln: T6vmvnv Fhj D1 F2 F2
65. Db00463
66. Cas-50-11-3
67. Ft-0692647
68. 5,5-diethyl-1-methyl-barbituric-acid
69. D01382
70. Q1176294
71. 5,5-diethyl-1-methyl-2,4,6-trioxo-hexahydropyrimidine
72. Z2205958937
73. 2,6(1h,3h,5h)-pyrimidinetrione, 5,5-diethyl-1-methyl-
74. 5,5-diethyl-1-methyl-2,4,6(1h,3h,5h)-pyrimidinetrione #
75. 5,5-diethyl-6-hydroxy-3-methyl-2,3,4,5-tetrahydropyrimidine-2,4-dione
76. Vok
| Molecular Weight | 198.22 g/mol |
|---|---|
| Molecular Formula | C9H14N2O3 |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 198.10044231 g/mol |
| Monoisotopic Mass | 198.10044231 g/mol |
| Topological Polar Surface Area | 66.5 Ų |
| Heavy Atom Count | 14 |
| Formal Charge | 0 |
| Complexity | 294 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Metharbital is used for the treatment of epilepsy.
Metharbital, a barbiturate, is used for the treatment of short term insomnia. It belongs to a group of medicines called central nervous system (CNS) depressants that induce drowsiness and relieve tension or nervousness. Little analgesia is conferred by barbiturates; their use in the presence of pain may result in excitation.
N - Nervous system
N03 - Antiepileptics
N03A - Antiepileptics
N03AA - Barbiturates and derivatives
N03AA30 - Metharbital
Metharbital binds at a distinct binding site associated with a Cl- ionopore at the GABAA receptor, increasing the duration of time for which the Cl- ionopore is open. The post-synaptic inhibitory effect of GABA in the thalamus is, therefore, prolonged. All of these effects are associated with marked decreases in GABA-sensitive neuronal calcium conductance (gCa). The net result of barbiturate action is acute potentiation of inhibitory GABAergic tone. Barbiturates also act through potent (if less well characterized) and direct inhibition of excitatory AMPA-type glutamate receptors, resulting in a profound suppression of glutamatergic neurotransmission.
