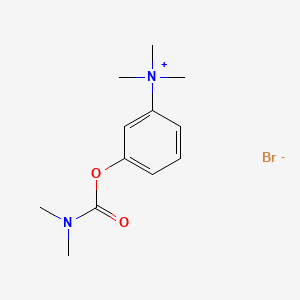

1. Bromide, Neostigmine
2. Methylsulfate, Neostigmine
3. Neostigmine
4. Neostigmine Methylsulfate
5. Polstigmine
6. Proserine
7. Prostigmin
8. Prostigmine
9. Prozerin
10. Synstigmin
11. Syntostigmine
1. 114-80-7
2. Eustigmin Bromide
3. Neoserine Bromide
4. Vagostigmin
5. Kirkstigmine Bromide
6. Syntostigmin Bromide
7. Neo-proserin
8. 3-((dimethylcarbamoyl)oxy)-n,n,n-trimethylbenzenaminium Bromide
9. Proserine Bromide
10. Stigmanol Bromide
11. Synstigmin Bromide
12. Leostigmine Bromide
13. Neostigmini Bromidum
14. Philostigmin Bromide
15. Prostigmin
16. Neo Proserine
17. Neostigmine Methyl Bromide
18. Bromure De Neostigmine
19. Bromuro De Neostigmina
20. Neostigmine (bromide)
21. Neoproserine
22. Neostigminebromide
23. (m-hydroxyphenyl)trimethylammonium Bromide Dimethylcarbamate
24. 3-[(dimethylcarbamoyl)oxy]-n,n,n-trimethylanilinium Bromide
25. Nsc-757233
26. 3-dimethylcarbamoxyphenyl Trimethyl Ammonium Bromide
27. 114-80-7 (bromide)
28. Mls000028387
29. [3-(dimethylcarbamoyloxy)phenyl]-trimethylazanium;bromide
30. 3-hydroxyphenyltrimethylammonium Bromide Dimethylcarbamic Ester
31. Chebi:179557
32. 005syp50g5
33. Eustigmin Bromide;neoserine Bromide
34. Smr000058591
35. Stigmosan Bromide
36. Benzenaminium, 3-(((dimethylamino)carbonyl)oxy)-n,n,n-trimethyl-, Bromide
37. Prostigmin Bromide
38. Dsstox_cid_21075
39. Dsstox_rid_79621
40. Prostigmine Bromide
41. Dsstox_gsid_41075
42. Synstigmini Bromidum
43. Vagostigmine Bromide
44. Syntostigmine Bromide
45. 3-(dimethylcarbamoyloxy)trimethylanilinium Ion Bromatum
46. Synthostigmine Bromide
47. Syntostigmin (tablet)
48. Synstigminbromid
49. Benzenaminium, 3-[[(dimethylamino)carbonyl]oxy]-n,n,n-trimethyl-, Bromide
50. Neostigmina Bromuro
51. Neostigmina Bromuro [dcit]
52. Neostigmini Bromidum [inn-latin]
53. Sr-01000000073
54. Ncgc00163240-01
55. Cas-114-80-7
56. Einecs 204-054-8
57. Bromure De Neostigmine [inn-french]
58. Bromuro De Neostigmina [inn-spanish]
59. Unii-005syp50g5
60. Vagostigmin (tn)
61. Prestwick_352
62. Mfcd00011795
63. Neostigmine Bromide [usp:inn:ban:jan]
64. Opera_id_1504
65. Carbamic Acid, Dimethyl-, Ester With (m-hydroxyphenyl)trimethylammonium Bromide
66. Neo Proserine [jan]
67. Schembl41128
68. Mls001146913
69. Chembl54126
70. Spectrum1500428
71. Lczc2572
72. Dtxsid9041075
73. Neostigmine Bromide (prostigmin)
74. Neostigmine Bromide [mi]
75. Hms500i07
76. Neostigmine Bromide [inn]
77. Neostigmine Bromide [jan]
78. Hms1569m18
79. Hms1920p15
80. Hms2091h18
81. Hms2096m18
82. Hms2235j11
83. Hms3262d13
84. Hms3372e02
85. Hms3713m18
86. Pharmakon1600-01500428
87. Neostigmine Bromide [vandf]
88. 3-(n,n-dimethylcarbamoyloxy)-n,n,n,-trimethylanilinium Bromide
89. Act05638
90. Hy-b0423
91. Neostigmine Bromide [mart.]
92. Neostigmine Bromide (jan/usp/inn)
93. Tox21_112037
94. Tox21_500816
95. Ccg-39110
96. Neostigmine Bromide [usp-rs]
97. Neostigmine Bromide [who-dd]
98. Neostigmine Bromide [who-ip]
99. Nsc757233
100. S2490
101. Akos015895725
102. Ammonium, (m-hydroxyphenyl)trimethyl-, Bromide, Dimethylcarbamate
103. Tox21_112037_1
104. Lp00816
105. Nsc 757233
106. Ncgc00015730-11
107. Ncgc00094149-01
108. Ncgc00094149-02
109. Ncgc00094149-03
110. Ncgc00094149-04
111. Ncgc00261501-01
112. Bs-14646
113. Neostigmine Bromide [ep Monograph]
114. Mls-0002855.p042
115. Neostigmine Bromide [usp Monograph]
116. Neostigmini Bromidum [who-ip Latin]
117. Eu-0100816
118. Ft-0651572
119. N0358
120. C08197
121. C71473
122. D00995
123. N 2001
124. A803245
125. Sr-01000000073-3
126. Sr-01000000073-5
127. Sr-01000000073-7
128. W-108598
129. (3-dimethylcarbamoyloxyphenyl)trimethylammonium Bromide
130. Q27105147
131. Neostigmine Bromide, >=98% (hplc And Titration), Powder
132. (3-hydroxyphenyl)trimethylammonium Bromide Dimethyl Carbamate
133. [3-[dimethylamino(oxo)methoxy]phenyl]-trimethylammonium Bromide
134. Neostigmine Bromide, European Pharmacopoeia (ep) Reference Standard
135. Neostigmine Bromide, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 303.20 g/mol |
|---|---|
| Molecular Formula | C12H19BrN2O2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 302.06299 g/mol |
| Monoisotopic Mass | 302.06299 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 17 |
| Formal Charge | 0 |
| Complexity | 246 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Cholinesterase Inhibitors
Drugs that inhibit cholinesterases. The neurotransmitter ACETYLCHOLINE is rapidly hydrolyzed, and thereby inactivated, by cholinesterases. When cholinesterases are inhibited, the action of endogenously released acetylcholine at cholinergic synapses is potentiated. Cholinesterase inhibitors are widely used clinically for their potentiation of cholinergic inputs to the gastrointestinal tract and urinary bladder, the eye, and skeletal muscles; they are also used for their effects on the heart and the central nervous system. (See all compounds classified as Cholinesterase Inhibitors.)
Parasympathomimetics
Drugs that mimic the effects of parasympathetic nervous system activity. Included here are drugs that directly stimulate muscarinic receptors and drugs that potentiate cholinergic activity, usually by slowing the breakdown of acetylcholine (CHOLINESTERASE INHIBITORS). Drugs that stimulate both sympathetic and parasympathetic postganglionic neurons (GANGLIONIC STIMULANTS) are not included here. (See all compounds classified as Parasympathomimetics.)
