
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Weekly News Recap #Phispers
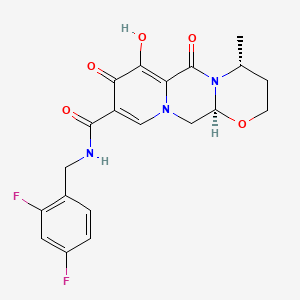

1. (4r,12as)-n-((2,4-difluorophenyl)methyl)-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide
2. (4r,9as)-5-hydroxy-4-methyl-6,10-dioxo-3,4,6,9,9a,10-hexahydro-2h-1-oxa-4a,8a-diaza-anthracene-7-carboxylic Acid- 2,4 Difluorobenzylamide
3. (4s,12ar)-n-((2,4-difluorophenyl)methyl)-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide
4. (4s,12as)-n-((2,4-difluorophenyl)methyl)-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide
5. Dolutegravir S,r Isomer
6. Dolutegravir S,s-isomer
7. Dolutegravir Sodium
8. Dolutegravir Sodium Monohydrate
9. Gsk 1349572a
10. Gsk-1349572
11. Gsk-1349572a
12. Gsk1349572a
13. S-gsk1349572
14. Tivicay
15. Tivicay Pd
1. 1051375-16-6
2. Gsk1349572
3. S/gsk1349572
4. Tivicay
5. Gsk-1349572
6. Gsk 1349572
7. Dolutegravir (gsk1349572)
8. S-349572
9. (4r,12as)-n-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide
10. Dolutegravir Dtg
11. Dolutegravir [usan]
12. Chebi:76010
13. (4r,12as)-n-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide
14. Dko1w9h7m1
15. Dolutegravir (usan)
16. 1051375-16-6 (free)
17. Tivicay (tn)
18. (3s,7r)-n-[(2,4-difluorophenyl)methyl]-11-hydroxy-7-methyl-9,12-dioxo-4-oxa-1,8-diazatricyclo[8.4.0.0^{3,8}]tetradeca-10,13-diene-13-carboxamide
19. (3s,7r)-n-[(2,4-difluorophenyl)methyl]-11-hydroxy-7-methyl-9,12-dioxo-4-oxa-1,8-diazatricyclo[8.4.0.03,8]tetradeca-10,13-diene-13-carboxamide
20. S-gsk1349572
21. Dolutegravir [usan:inn]
22. Unii-dko1w9h7m1
23. Soltegravir
24. Hsdb 8152
25. 3s3m
26. 3s3n
27. 3s3o
28. (4r,12as)-n-[(2,4-difluorophenyl)methyl]-7-hydroxy-4-methyl-6,8-dioxo-3,4,12,12a-tetrahydro-2h-pyrido[[?]:[?]]pyrazino[[?]][1,3]oxazine-9-carboxamide
29. Dolutegravir [mi]
30. Dolutegravir [inn]
31. Dolutegravir [vandf]
32. Schembl82071
33. Mls006011137
34. Dolutegravir [who-dd]
35. Gtpl7365
36. Chembl1229211
37. Dtxsid90909356
38. Ex-a1695
39. Bdbm50062551
40. Mfcd20488027
41. S2667
42. Zinc58581064
43. Akos025396657
44. S/gsk-1349572
45. Bcp9000620
46. Ccg-268876
47. Cs-0454
48. Db08930
49. Ncgc00346629-01
50. Ncgc00346629-02
51. 2h-pyrido(1',2':4,5)pyrazino(2,1-b)(1,3)oxazine-9-carboxamide, N-((2,4-difluorophenyl)methyl)-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-, (4r,12as)-
52. Ac-28371
53. As-75277
54. Hy-13238
55. Smr004702915
56. S/gsk1349572,gsk1349572
57. D10066
58. A854801
59. Q937224
60. J-501471
61. (3s,7r)-n-[(2,4-difluorophenyl)methyl]-11-hydroxy-7-methyl-9,12-dioxo-4-oxa-1,8-diazatricyclo[8.4.0.0(3),?]tetradeca-10,13-diene-13-carboxamide
62. (4r,12.alpha.s)-n-((2,4-difluorophenyl)methyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12.alpha.-hexahydro-2h-pyrido(1',2':4,5)pyrazino(2,1-.beta.)(1,3)oxazine-9-carboxamide
63. (4r,12as)-n-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-[1,3]oxazino[3,2-a]pyrido[1,2-d]pyrazine-9-carboxamide
64. (4r,12as)-n-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxamide
65. (4r,12as)-n-(2,4-difluorobenzyl)-7-hydroxy-4-methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2h-pyrido[1,2:4,5]pyrazino[2,1-b][1,3]oxazine-9-carboxamide
66. (4r,12as)-n-[(2,4-difluorophenyl)methyl]-3,4,6,8,12,12a-hexahydro-7-hydroxy-4-methyl-6,8-dioxo-2h-pyrido[1',2':4,5]pyrazino[2,1-b][1 ,3]oxazine-9-carboxamide
67. (4r,12as)-n-[(2,4-difluorophenyl)methyl]-7-hydroxy-4-methyl-6,8-dioxo-3,4,12,12a-tetrahydro-2h-pyrido[5,6]pyrazino[2,6-b][1,3]oxazine-9-carboxamide
| Molecular Weight | 419.4 g/mol |
|---|---|
| Molecular Formula | C20H19F2N3O5 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 3 |
| Exact Mass | 419.12927704 g/mol |
| Monoisotopic Mass | 419.12927704 g/mol |
| Topological Polar Surface Area | 99.2 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 829 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
HIV Integrase Inhibitors
National Library of Medicine's Medical Subject Headings. Dolutegravir. Online file (MeSH, 2014). Available from, as of November 19, 2013: https://www.nlm.nih.gov/mesh/2014/mesh_browser/MBrowser.html
The recommended dose of TIVICAY in pediatric patients aged 12 years and older and weighing at least 40 kg is 50 mg administered orally once daily. If efavirenz, fosamprenavir/ritonavir, tipranavir/ritonavir, or rifampin are coadministered, the recommended dose of TIVICAY is 50 mg twice daily. Safety and efficacy of TIVICAY have not been established in pediatric patients younger than 12 years or weighing less than 40 kg, or in pediatric patients who are INSTI-experienced with documented or clinically suspected resistance to other INSTIs (raltegravir, elvitegravir).
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
TIVICAY (dolutegravir) is indicated in combination with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and children aged 12 years and older and weighing at least 40 kg. The following should be considered prior to initiating treatment with TIVICAY: Poor virologic response was observed in subjects treated with TIVICAY 50 mg twice daily with an integrase strand transfer inhibitor (INSTI)-resistance Q148 substitution plus 2 or more additional INSTI-resistance substitutions, including L74I/M, E138A/D/K/T, G140A/S, Y143H/R, E157Q, G163E/K/Q/R/S, or G193E/R.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Renal clearance of unchanged drug is a minor pathway of elimination for dolutegravir. In a trial comparing 8 subjects with severe renal impairment (CrCl <30 mL/min) with 8 matched healthy controls, AUC, Cmax, and C24 of dolutegravir were decreased by 40%, 23%, and 43%, respectively, compared with those in matched healthy subjects. The cause of this decrease is unknown. Population pharmacokinetic analysis using data from SAILING and VIKING-3 trials indicated that mild and moderate renal impairment had no clinically relevant effect on the exposure of dolutegravir. No dosage adjustment is necessary for treatment-naive or treatment-experienced and INSTI-naive patients with mild, moderate, or severe renal impairment or for INSTI-experienced patients (with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance) with mild or moderate renal impairment. Caution is warranted for INSTI-experienced patients (with certain INSTI-associated resistance substitutions or clinically suspected INSTI resistance [see Microbiology (12.4)]) with severe renal impairment, as the decrease in dolutegravir concentrations may result in loss of therapeutic effect and development of resistance to TIVICAY or other coadministered antiretroviral agents. Dolutegravir has not been studied in patients requiring dialysis.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Dolutegravir is primarily metabolized and eliminated by the liver. In a trial comparing 8 subjects with moderate hepatic impairment (Child-Pugh Score B) with 8 matched healthy controls, exposure of dolutegravir from a single 50-mg dose was similar between the 2 groups. No dosage adjustment is necessary for patients with mild to moderate hepatic impairment (Child-Pugh Score A or B). The effect of severe hepatic impairment (Child-Pugh Score C) on the pharmacokinetics of dolutegravir has not been studied. Therefore, TIVICAY is not recommended for use in patients with severe hepatic impairment.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
The Centers for Disease Control and Prevention recommend that HIV-1-infected mothers in the United States not breastfeed their infants to avoid risking postnatal transmission of HIV-1 infection. Studies in lactating rats and their offspring indicate that dolutegravir was present in rat milk. It is not known whether dolutegravir is excreted in human milk. Because of both the potential for HIV transmission and the potential for adverse reactions in nursing infants, mothers should be instructed not to breastfeed if they are receiving TIVICAY.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Pregnancy Category B. There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, and dolutegravir was shown to cross the placenta in animal studies, this drug should be used during pregnancy only if clearly needed.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Dolutegravir (TIVICAY) should not be used with etravirine without coadministration of atazanavir/ritonavir, darunavir/ritonavir, or lopinavir/ritonavir.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy, including TIVICAY. During the initial phase of combination antiretroviral treatment, patients whose immune systems respond may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis), which may necessitate further evaluation and treatment. Autoimmune disorders (such as Graves' disease, polymyositis, and Guillain-Barre syndrome) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable and can occur many months after initiation of treatment.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
For more Drug Warnings (Complete) data for Dolutegravir (8 total), please visit the HSDB record page.
Dolutegravir is indicated in combination with other antiretroviral agents for the treatment of patients with HIV-1 infection that comply with the characteristics of being adults or children aged 12 years and older and present at least a weight of 40 kg. The FDA combination therapy approval of dolutegravir and rilpivirine is indicated for adults with HIV-1 infections whose virus is currently suppressed (< 50 copies/ml) on a stable regimen for at least six months, without history of treatment failure and no known substitutions associated to resistance to any of the two components of the therapy.
FDA Label
Tivicay is indicated in combination with other anti-retroviral medicinal products for the treatment of Human Immunodeficiency Virus (HIV) infected adults, adolescents and children of at least 6 years of age or older and weighing at least 14 kg.
Tivicay is indicated in combination with other anti-retroviral medicinal products for the treatment of Human Immunodeficiency Virus (HIV) infected adults, adolescents and children of at least 4 weeks of age or older and weighing at least 3 kg.
HIV-1 infected subjects on dolutegravir monotherapy demonstrated rapid and dose-dependent reduction of antiviral activity with declines of HIV-1 RNA copies per ml. The antiviral response was maintained for 3 to 4 days after the last dose. The sustained response obtained in clinical trials indicates that dolutegravir has a tight binding and longer dissociative half-life providing it a high barrier to resistance. The combination therapy (ripivirine and dolutegravir) presented the same viral suppression found in previous three-drug therapies without integrase strand transfer inhibitor mutations or rilpivirine resistance.
HIV Integrase Inhibitors
Inhibitors of HIV INTEGRASE, an enzyme required for integration of viral DNA into cellular DNA. (See all compounds classified as HIV Integrase Inhibitors.)
J05AX12
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AJ - Integrase inhibitors
J05AJ03 - Dolutegravir
Absorption
When 50 mg of dolutegravir once daily was orally administered to HIV-1 infected adults, the AUC, Cmax, and Cmin is 53.6 mcg h/mL, 3.67 mcg/mL, and 1.11 mcg/mL, respectively. The peak plasma concentration was observed 2 to 3 hours post-dose. Steady state is achieved within approximately 5 days with average accumulation ratios for AUC, Cmax, and C24h ranging from 1.2 to 1.5. When 50 mg once daily is given to pediatric patients (12 to < 18 years and weighing 40 kg) the Cmax, AUC, and C24 is 3.49 mcg/mL, 46 mcg.h/mL, and 0.90 mcg/mL respectively.
Route of Elimination
When a single oral dose of dolutegravir is given, nearly all complete dose is recovered in a proportion of 53% excreted unchanged in the feces and 31% excreted in urine. The renal eliminated recovered dose consists of ether glucuronide of dolutegravir (18.9%), a metabolite formed by oxidation at the benzylic carbon (3.0%), a hydrolytic N-dealkylation product (3.6%) and unchanged drug (< 1%).
Volume of Distribution
The administration of a dose of 50 mg of dolutegravir presents an apparent volume of distribution of 17.4 L. The median dolutegravir concentration in CSF was 18 ng/mL after 2 weeks of treatment.
Clearance
The apparent clearance rate of dultegravir is 1.0 L/h.
... After a single oral dose of [14C] dolutegravir, 53% of the total oral dose was excreted unchanged in feces. Thirty-one percent of the total oral dose was excreted in urine, represented by an ether glucuronide of dolutegravir (18.9% of total dose), a metabolite formed by oxidation at the benzylic carbon (3.0% of total dose), and its hydrolytic N-dealkylation product (3.6% of total dose). Renal elimination of unchanged drug was low (<1% of the dose).
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Dolutegravir is highly bound (=98.9%) to human plasma proteins based on in vivo data and binding is independent of plasma concentration of dolutegravir. The apparent volume of distribution (Vd/F) following 50-mg once-daily administration is estimated at 17.4 L based on a population pharmacokinetic analysis.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Food increased the extent of absorption and slowed the rate of absorption of dolutegravir. Low-, moderate-, and high-fat meals increased dolutegravir AUC(0-8) by 33%, 41%, and 66%; increased Cmax by 46%, 52%, and 67%; and prolonged Tmax to 3, 4, and 5 hours from 2 hours under fasted conditions, respectively.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Following oral administration of dolutegravir, peak plasma concentrations were observed 2 to 3 hours postdose. With once-daily dosing, pharmacokinetic steady state is achieved within approximately 5 days with average accumulation ratios for AUC, Cmax, and C24 h ranging from 1.2 to 1.5. Dolutegravir plasma concentrations increased in a less than dose-proportional manner above 50 mg. Dolutegravir is a P-glycoprotein substrate in vitro. The absolute bioavailability of dolutegravir has not been established.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Dolutegravir is highly metabolized through three main pathways and it forms no long-lived metabolites. The first pathway is defined by the glucuronidation by UGT1A1, the second pathway by carbon oxidation by CYP3A4 and the third pathway is what appears to be a sequential oxidative defluorination and glutathione conjugation. The main metabolite found in blood plasma is the ether glucuronide form (M2) and its chemical properties disrupt its ability to bind metal ions, therefore, it is inactive.
Dolutegravir is primarily metabolized via UGT1A1 with some contribution from CYP3A. ... ether glucuronide of dolutegravir (18.9% of total dose), a metabolite formed by oxidation at the benzylic carbon (3.0% of total dose), and its hydrolytic N-dealkylation product (3.6% of total dose). ...
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
The half-life of dolutegravir is 14 hours.
Dolutegravir has a terminal half-life of approximately 14 hours and an apparent clearance (CL/F) of 1.0 L/h based on population pharmacokinetic analyses.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922
Dolutegravir is an HIV-1 antiviral agent. It inhibits HIV integrase by binding to the active site and blocking the strand transfer step of retroviral DNA integration in the host cell. The strand transfer step is essential in the HIV replication cycle and results in the inhibition of viral activity. Dolutegravir has a mean EC50 value of 0.5 nM (0.21 ng/mL) to 2.1 nM (0.85 ng/mL) in peripheral blood mononuclear cells (PBMCs) and MT-4 cells.
Dolutegravir inhibits HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral deoxyribonucleic acid (DNA) integration which is essential for the HIV replication cycle. Strand transfer biochemical assays using purified HIV-1 integrase and pre-processed substrate DNA resulted in IC50 values of 2.7 nM and 12.6 nM.
US Natl Inst Health; DailyMed. Current Medication Information for TIVICAY (dolutegravir sodium) tablet, film coated TIVICAY (dolutegravir) Tablets for Oral Use (Initial U.S. Approval: 2013). Available from, as of November 22, 2013: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=63df5af3-b8ac-4e76-9830-2dbb340af922

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Gradovir
Dosage Form : TAB
Dosage Strength : 50mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Vulante
Dosage Form : FCT
Dosage Strength : 50mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
Regulatory Info :
Registration Country : India
Brand Name : Dolutegravir
Dosage Form : Tablet
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Regulatory Info :
Registration Country : India
Dolutegravir; Lamivudine; Tenofovir Disoproxil Fumarate
Brand Name : Dolutegravir; Lamivudine; Tenofovir Disoproxil Fumarate
Dosage Form : Tablet
Dosage Strength : 50MG; 300MG; 300MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Regulatory Info :
Registration Country : India
Tenofovir Disoproxil Fumarate; Lamivudine; Dolutegravir
Brand Name : Tenofovir Disoproxil Fumarate; Lamivudine; Dolutegravir
Dosage Form : Tablet
Dosage Strength : 300MG; 300MG; 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Regulatory Info :
Registration Country : India
Dolutegravir; Lamivudine; Tenofovir Disoproxil Fumarate
Brand Name : Dolutegravir; Lamivudine; Tenofovir Disoproxil Fumarate
Dosage Form : Tablet
Dosage Strength : 600MG; 200MG; 300MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Lendofil
Dosage Form : FCT
Dosage Strength : 50mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : RX
Registration Country : USA
Brand Name : TIVICAY
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 50MG BASE
Packaging :
Approval Date : 2013-08-12
Application Number : 204790
Regulatory Info : RX
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : TIVICAY
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 10MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 2016-06-09
Application Number : 204790
Regulatory Info : DISCN
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : TIVICAY
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 25MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 2016-06-09
Application Number : 204790
Regulatory Info : DISCN
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : DOLUTEGRAVIR SODIUM
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG
Approval Date :
Application Number : 209658
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : DOLUTEGRAVIR
Dosage Form : FILM;ORAL
Dosage Strength : 5MG
Approval Date :
Application Number : 215319
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : DOLUTEGRAVIR
Dosage Form : FILM;ORAL
Dosage Strength : 10MG
Approval Date :
Application Number : 215319
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : DOLUTEGRAVIR SODIUM
Dosage Form : TABLET; ORAL SUSPENSION
Dosage Strength : 10MG
Approval Date :
Application Number : 214566
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : DOLUTEGRAVIR SODIUM
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG
Approval Date :
Application Number : 212179
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
DOLUTEGRAVIR; LAMIVUDINE; TENOFOVIR DISOPROXIL FUMARATE
Brand Name : DOLUTEGRAVIR, LAMIVUDINE AND TENOFOVIR DISOPROXIL FUMARATE
Dosage Form : TABLET; ORAL
Dosage Strength : 50MG/300MG/300MG
Approval Date :
Application Number : 212303
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
Brand Name : DOLUTEGRAVIR SODIUM
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG
Approval Date :
Application Number : 209602
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD :
TE Code :
DOLUTEGRAVIR; LAMIVUDINE; TENOFOVIR DISOPROXIL FUMARATE
Brand Name : DOLUTEGRAVIR, LAMIVUDINE, AND TENOFOVIR DISOPROXIL FUMARATE
Dosage Form : TABLET;ORAL
Dosage Strength : 50MG;300MG;300MG
Approval Date :
Application Number : 209670
RX/OTC/DISCN :
RLD :
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
Brand Name : TIVICAY
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 50MG BASE
Approval Date : 2013-08-12
Application Number : 204790
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
RLD : Yes
TE Code :
DOLUTEGRAVIR SODIUM; RILPIVIRINE HYDROCHLORIDE
Brand Name : JULUCA
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 50MG BASE;EQ 25MG BASE
Approval Date : 2017-11-21
Application Number : 210192
RX/OTC/DISCN : RX
RLD : Yes
TE Code :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Tivicay
Dosage Form : tablet
Dosage Strength : 50 mg
Packaging : 30
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Dolutegravir + abacavir + lamivudine
Brand Name : Triumeq
Dosage Form : Tablet
Dosage Strength : 50MG
Packaging : 30
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Juluca
Dosage Form : Tablet
Dosage Strength : 50MG
Packaging : 30
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Australia
Brand Name : Dovato
Dosage Form : Tablet
Dosage Strength : 50MG
Packaging : 30
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Australia

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Regulatory Info :
Registration Country : India
Brand Name : Dolutegravir
Dosage Form : Tablet
Dosage Strength : 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging :
Regulatory Info :
Dosage : Tablet
Dosage Strength : 50MG
Brand Name : Dolutegravir
Approval Date :
Application Number :
Registration Country : India
Regulatory Info :
Registration Country : India
Dolutegravir; Lamivudine; Tenofovir Disoproxil Fumarate
Brand Name : Dolutegravir; Lamivudi...
Dosage Form : Tablet
Dosage Strength : 50MG; 300MG; 300MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging :
Regulatory Info :
Dolutegravir; Lamivudine; Tenofovir Disoproxil Fumarate
Dosage : Tablet
Dosage Strength : 50MG; 300MG; 300MG
Brand Name : Dolutegravir; Lamivudi...
Approval Date :
Application Number :
Registration Country : India
Regulatory Info :
Registration Country : India
Tenofovir Disoproxil Fumarate; Lamivudine; Dolutegravir
Brand Name : Tenofovir Disoproxil F...
Dosage Form : Tablet
Dosage Strength : 300MG; 300MG; 50MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging :
Regulatory Info :
Tenofovir Disoproxil Fumarate; Lamivudine; Dolutegravir
Dosage : Tablet
Dosage Strength : 300MG; 300MG; 50MG
Brand Name : Tenofovir Disoproxil F...
Approval Date :
Application Number :
Registration Country : India
Regulatory Info :
Registration Country : India
Dolutegravir; Lamivudine; Tenofovir Disoproxil Fumarate
Brand Name : Dolutegravir; Lamivudi...
Dosage Form : Tablet
Dosage Strength : 600MG; 200MG; 300MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India
Packaging :
Regulatory Info :
Dolutegravir; Lamivudine; Tenofovir Disoproxil Fumarate
Dosage : Tablet
Dosage Strength : 600MG; 200MG; 300MG
Brand Name : Dolutegravir; Lamivudi...
Approval Date :
Application Number :
Registration Country : India
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : India
Brand Name : DTV 50
Dosage Form : Tablet
Dosage Strength : 50MG
Packaging : Jar pack of 30's
Approval Date :
Application Number :
Regulatory Info :
Registration Country : India

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Packaging : Jar pack of 30's
Regulatory Info :
Dosage : Tablet
Dosage Strength : 50MG
Brand Name : DTV 50
Approval Date :
Application Number :
Registration Country : India

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Capsule
Grade : Oral
Brand Name : Sodium Stearyl Fumarate G...
Application : Lubricants & Glidants
Excipient Details : Sodium Stearyl Fumarate is used as a lubricant in OSDs to reduce the friction and the adhesion. It also provides tablet strength & disintegration.
Pharmacopoeia Ref : USP, EP, ICH, Q7GMP
Technical Specs : NA
Ingredient(s) : Sodium Stearyl Fumarate
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Taste Masking
Excipient Details : Mannitol is used as a filler, bulking agent and taste masking agent in ODT formulations such as tablets.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Controlled & Modified Release
Excipient Details : ACTILLETS are microcrystalline cellulose spheres developed using advanced drug delivery technology to enable effective loading & coating of particles.
Pharmacopoeia Ref : NA
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Application : Disintegrants & Superdisintegrants
Excipient Details : Mannogem 2080 exhibits excellent flow, disintegration and compression properties.
Dosage Form : Orodispersible Tablet
Grade : Oral
Dosage Form : Orodispersible Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Brand Name : Titanium dioxide PRETIOX ...
Application : Coloring Agents
Excipient Details : Titanium dioxide Pretiox AV01FG is used as a coloring and coating agent in oral solid dosage forms such as capsules, tablets, granules, and pellets.
Pharmacopoeia Ref : Fami-QS, Kosher, Halal, OHSAS ...
Technical Specs : Ti 59.95% and O 40.05%
Ingredient(s) : Titanium Dioxide
Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Application : Controlled & Modified Release
Excipient Details : Espheres EM can be used as an inert base for modified release formulations promoting uniformity of release profile.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Tablet
Grade : Oral
Application : Coating Systems & Additives
Excipient Details : Instacoat EMB is an excellent moisture barrier which reduces degradation of moisture sensitive APIs.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Brand Name : Croscarmellose Sodium
Application : Disintegrants & Superdisintegrants
Excipient Details : Cross Carmellose Sodium is fast disintegrating agent or a super-disintegrant used in pharmaceutical tablet formulations.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Croscarmellose Sodium
Dosage Form : Tablet
Grade : Oral
Brand Name : Microcrystalline Cellulos...
Application : Fillers, Diluents & Binders
Excipient Details : Microcrystalline Cellulose is most commonly used filler and binder in drug formulations, together with Lactose.
Pharmacopoeia Ref : Not Available
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Brand Name : Microlex® LCM 120.s
Application : Granulation
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Sieved; Also Available as Microlex® LCM 180.s, Microlex® LCM 220.s
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Oral
Brand Name : Microlex® LCM 80.m
Application : Granulation
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Milled; Also Available as Microlex® LCM 180.m, Microlex® LCM 200.m
Ingredient(s) : Lactose Monohydrate
Dosage Form : Tablet
Grade : Oral
Brand Name : Microlex® MCC 101
Application : Granulation
Excipient Details : Tablets made from these granules are typically easily disintegrated using conventional super disintegrates even when hard tablets are compressed.
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Tablet
Grade : Oral
Brand Name : Microlex® MCC 102
Application : Direct Compression
Pharmacopoeia Ref : Monograph- USP/NF, JP/JPE
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Capsule
Grade : Topical, Oral
Brand Name : Microlex® MLP 520
Application : Fillers, Diluents & Binders
Excipient Details : Use in a wide range of oral applications such as wet or dry granulation, excipient of choice for flash release forms.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Available in different particle size as 25.µ, 50.µ, 180.µ
Ingredient(s) : Mannitol
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Excipient Details : Most popular excipient for the production of tablets and capsules. Offering an efficient and low dosage in capsules.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-6-10 m2/g; Particle Size-7-11 µm
Ingredient(s) : Magnesium Stearate
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Excipient Details : Higher specific surface area and a smaller median particle size. This product is preferred for more critical and very fine herbal formulations.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-8-12 m2/g; Particle Size-5-9 µm
Ingredient(s) : Magnesium Stearate
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Specific Surface Area-6-8 m2/g; Particle Size-7-11 µm
Ingredient(s) : Magnesium Stearate
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Not Available
Ingredient(s) : Magnesium Stearate
Dosage Form : Tablet
Grade : Oral
Application : Lubricants & Glidants
Excipient Details : Meets Kosher and Halal preparations in Jewish and Arab cultures. Qualified in high specification standards requested by European formulators.
Pharmacopoeia Ref : Monograph- Ph.Eur, USP/NF
Technical Specs : Not Available
Ingredient(s) : Magnesium Stearate
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Dosage Form : Injectable / Parenteral
Grade : Oral, Parenteral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipient Details : Helps to manufacture Oral Dosage and Nutraceutical forms by acting as a filler-binder while serving as a fibre source for your customers.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Tablet
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipient Details : Its range offers a unique blend of exceptional physical and chemical stability and no hygroscopicity.
Dosage Form : Injectable / Parenteral
Grade : Not Available
Brand Name : PEARLITOL® BIOPHARMA
Application : Parenteral
Excipient Details : Its range offers a unique blend of exceptional physical and chemical stability and no hygroscopicity.
Excipients Web Link
Dosage Form : Tablet
Grade : Not Available
Application : Fillers, Diluents & Binders
Excipients Web Link
Dosage Form : Tablet
Grade : Not Available
Application : Disintegrants & Superdisintegrants
Excipient Details : It is a superdisintegrant that provides an efficient disintegration at low level of use
Dosage Form : Cream / Lotion / Ointment
Grade : Not Available
Application : Thickeners and Stabilizers
Excipient Details : A co-processed product used as a secondary stabilizer and suspension agent in semi-solides formulas.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Cream / Lotion / Ointment
Grade : Not Available
Application : Thickeners and Stabilizers
Excipient Details : A co-processed product used as a secondary stabilizer and suspension agent in semi-solides formulas.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Cream / Lotion / Ointment
Grade : Not Available
Application : Thickeners and Stabilizers
Excipient Details : A co-processed product used as a secondary stabilizer and suspension agent in semi-solides formulas.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Cream / Lotion / Ointment
Grade : Not Available
Brand Name : TABULOSE® SC 591F
Application : Thickeners and Stabilizers
Excipient Details : A co-processed product used as a secondary stabilizer and suspension agent in semi-solides formulas.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
Dosage Form : Cream / Lotion / Ointment
Grade : Not Available
Application : Thickeners and Stabilizers
Excipient Details : A co-processed product used as a secondary stabilizer and suspension agent in semi-solides formulas.
Pharmacopoeia Ref : EP/USP/JP
Technical Specs : Not Available
Ingredient(s) : Microcrystalline Cellulose Excipients
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ABOUT THIS PAGE
16
PharmaCompass offers a list of Dolutegravir Sodium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Dolutegravir Sodium manufacturer or Dolutegravir Sodium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Dolutegravir Sodium manufacturer or Dolutegravir Sodium supplier.
PharmaCompass also assists you with knowing the Dolutegravir Sodium API Price utilized in the formulation of products. Dolutegravir Sodium API Price is not always fixed or binding as the Dolutegravir Sodium Price is obtained through a variety of data sources. The Dolutegravir Sodium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Dolutegravir Sodium manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Dolutegravir Sodium, including repackagers and relabelers. The FDA regulates Dolutegravir Sodium manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Dolutegravir Sodium API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Dolutegravir Sodium manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Dolutegravir Sodium supplier is an individual or a company that provides Dolutegravir Sodium active pharmaceutical ingredient (API) or Dolutegravir Sodium finished formulations upon request. The Dolutegravir Sodium suppliers may include Dolutegravir Sodium API manufacturers, exporters, distributors and traders.
click here to find a list of Dolutegravir Sodium suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Dolutegravir Sodium DMF (Drug Master File) is a document detailing the whole manufacturing process of Dolutegravir Sodium active pharmaceutical ingredient (API) in detail. Different forms of Dolutegravir Sodium DMFs exist exist since differing nations have different regulations, such as Dolutegravir Sodium USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Dolutegravir Sodium DMF submitted to regulatory agencies in the US is known as a USDMF. Dolutegravir Sodium USDMF includes data on Dolutegravir Sodium's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Dolutegravir Sodium USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Dolutegravir Sodium suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Dolutegravir Sodium Drug Master File in Korea (Dolutegravir Sodium KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Dolutegravir Sodium. The MFDS reviews the Dolutegravir Sodium KDMF as part of the drug registration process and uses the information provided in the Dolutegravir Sodium KDMF to evaluate the safety and efficacy of the drug.
After submitting a Dolutegravir Sodium KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Dolutegravir Sodium API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Dolutegravir Sodium suppliers with KDMF on PharmaCompass.
A Dolutegravir Sodium written confirmation (Dolutegravir Sodium WC) is an official document issued by a regulatory agency to a Dolutegravir Sodium manufacturer, verifying that the manufacturing facility of a Dolutegravir Sodium active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Dolutegravir Sodium APIs or Dolutegravir Sodium finished pharmaceutical products to another nation, regulatory agencies frequently require a Dolutegravir Sodium WC (written confirmation) as part of the regulatory process.
click here to find a list of Dolutegravir Sodium suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Dolutegravir Sodium as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Dolutegravir Sodium API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Dolutegravir Sodium as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Dolutegravir Sodium and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Dolutegravir Sodium NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Dolutegravir Sodium suppliers with NDC on PharmaCompass.
Dolutegravir Sodium Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Dolutegravir Sodium GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Dolutegravir Sodium GMP manufacturer or Dolutegravir Sodium GMP API supplier for your needs.
A Dolutegravir Sodium CoA (Certificate of Analysis) is a formal document that attests to Dolutegravir Sodium's compliance with Dolutegravir Sodium specifications and serves as a tool for batch-level quality control.
Dolutegravir Sodium CoA mostly includes findings from lab analyses of a specific batch. For each Dolutegravir Sodium CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Dolutegravir Sodium may be tested according to a variety of international standards, such as European Pharmacopoeia (Dolutegravir Sodium EP), Dolutegravir Sodium JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Dolutegravir Sodium USP).

