



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
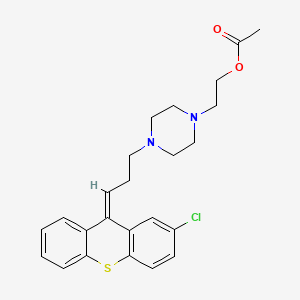

1. (z)-4-(3-(2-chlorothioxanthen-9-ylidene)propyl)-1-piperazineethanol Acetate
2. Clopenthixol Acetate Ester
3. Clopixol-acuphase
1. 85721-05-7
2. 2-[4-[(3z)-3-(2-chlorothioxanthen-9-ylidene)propyl]piperazin-1-yl]ethyl Acetate
3. 349s2zhf05
4. Clopenthixol Acetate Ester
5. Clopixol Acuphase
6. Clopixol-acuphase
7. Cisordinol-acutard (tn)
8. Einecs 288-415-5
9. Unii-349s2zhf05
10. Schembl1651216
11. Dtxsid00235032
12. Chebi:135722
13. Zinc30691716
14. (z)-4-(3-(2-chlorothioxanthen-9-ylidene)propyl)-1-piperazineethanol Acetate
15. Akos015841102
16. Zuclopenthixol Acetate [mart.]
17. Zuclopenthixol Acetate [who-dd]
18. (z)-4-(3-(2-chloro-9h-thioxanthen-9-ylidene)propyl)piperazine-1-ethyl Acetate
19. D08691
20. Q27256348
21. 4-[3-[(z)-2-chloro-9h-thioxanthen-9-ylidene]propyl]-1-piperazineethanol Acetate
| Molecular Weight | 443.0 g/mol |
|---|---|
| Molecular Formula | C24H27ClN2O2S |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 7 |
| Exact Mass | 442.1481770 g/mol |
| Monoisotopic Mass | 442.1481770 g/mol |
| Topological Polar Surface Area | 58.1 Ų |
| Heavy Atom Count | 30 |
| Formal Charge | 0 |
| Complexity | 608 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)


