


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
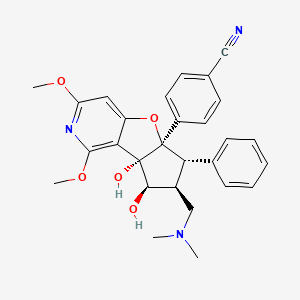

1. 2098191-53-6
2. Eft226
3. Eft-226
4. Zotatifin [inn]
5. Zotatifin [usan]
6. 2ewn8z05cn
7. 4-((5ar,6s,7s,8r,8as)-7-((dimethylamino)methyl)-8,8a-dihydroxy-1,3-dimethoxy-6-phenyl-6,7,8,8a-tetrahydro-5ah-cyclopenta[4,5]furo[3,2-c]pyridin-5a-yl)benzonitrile
8. 4-((5ar,6s,7s,8r,8as)-7-((dimethylamino)methyl)-8,8a-dihydroxy-1,3-dimethoxy-6-phenyl-6,7,8,8a-tetrahydro-5ah-cyclopenta(4,5)furo(3,2-c)pyridin-5a-yl)benzonitrile
9. Benzonitrile, 4-((5ar,6s,7s,8r,8as)-7-((dimethylamino)methyl)-6,7,8,8a-tetrahydro-8,8a-dihydroxy-1,3-dimethoxy-6-phenyl-5ah-cyclopenta(4,5)furo(3,2-c)pyridin-5a-yl)-
10. Zotafin
11. Rel-zotatifin
12. Zotatifin (usan/inn)
13. Unii-2ewn8z05cn
14. Zotatifin [who-dd]
15. Chembl4303782
16. Schembl18864788
17. Dtxsid001022536
18. Ex-a4623
19. Bdbm50540861
20. Nsc818001
21. Nsc828584
22. Who 10998
23. Nsc-818001
24. Nsc-828584
25. Hy-112163
26. Cs-0043584
27. D11837
28. A937238
29. 4-[(2s,3r,4s,5s,6r)-4-[(dimethylamino)methyl]-2,3-dihydroxy-10,12-dimethoxy-5-phenyl-7-oxa-11-azatricyclo[6.4.0.02,6]dodeca-1(12),8,10-trien-6-yl]benzonitrile
| Molecular Weight | 487.5 g/mol |
|---|---|
| Molecular Formula | C28H29N3O5 |
| XLogP3 | 2.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 487.21072103 g/mol |
| Monoisotopic Mass | 487.21072103 g/mol |
| Topological Polar Surface Area | 108 Ų |
| Heavy Atom Count | 36 |
| Formal Charge | 0 |
| Complexity | 819 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 5 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


