
Synopsis
Synopsis
0
API Suppliers
0
USDMF
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDF Dossiers
0
FDA Orange Book
0
Europe
0
Canada
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
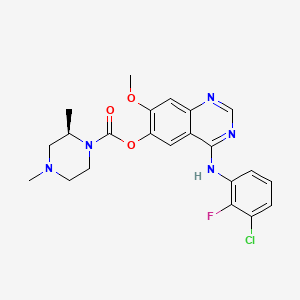

1. 4-((3-chloro-2-fluorophenyl)amino)-7-methoxyquinazolin-6-yl 2,4-dimethylpiperazine-1-carboxylate
2. Azd3759
1. Azd3759
2. 1626387-80-1
3. Zorifertinib
4. Azd-3759
5. Azd 3759
6. Chembl3623290
7. 67sx9h68w2
8. 4-[(3-chloro-2-fluorophenyl)amino]-7-methoxyquinazolin-6-yl (2r)-2,4-dimethylpiperazine-1-carboxylate
9. (2r)-2,4-dimethyl-1-piperazinecarboxylic Acid, 4-[(3-chloro-2-fluorophenyl)amino]-7-methoxy-6-quinazolinyl Ester
10. [4-(3-chloro-2-fluoroanilino)-7-methoxyquinazolin-6-yl] (2r)-2,4-dimethylpiperazine-1-carboxylate
11. 4-((3-chloro-2-fluorophenyl)amino)-7-methoxyquinazolin-6-yl (2r)-2,4-dimethylpiperazine-1-carboxylate
12. Zorifertinib [inn]
13. Unii-67sx9h68w2
14. Zorifertinib [who-dd]
15. Schembl16010006
16. Gtpl10456
17. Ex-a720
18. Amy10302
19. Bdbm50123453
20. Mfcd29058564
21. Nsc788121
22. Nsc800978
23. S7971
24. Akos027327321
25. Zinc221149242
26. Ccg-269329
27. Cs-5029
28. Db14795
29. Nsc-788121
30. Nsc-800978
31. Compound 1m [pmid: 26313252]
32. Ncgc00483922-01
33. Ncgc00483922-02
34. Ac-29957
35. As-74755
36. Hy-18750
37. A882818
38. (2r)-2,4-dimethyl-1-piperazinecarboxylic Acid 4-[(3-chloro-2-fluorophenyl)amino]-7-methoxy-6-quinazolinyl Ester
39. (2r)-2,4-dimethylpiperazine-1-carboxylic Acid (4-(2-fluoro-3-chloroanilino)-7-methoxyquinazoline-6-yl) Ester
40. (r)-4-(3-chloro-2-fluorophenylamino)-7-methoxyquinazolin-6-yl 2,4-dimethylpiperazine-1-carboxylate
41. 1-piperazinecarboxylic Acid, 2,4-dimethyl-, 4-((3-chloro-2-fluorophenyl)amino)-7-methoxy-6-quinazolinyl Ester, (2r)-
| Molecular Weight | 459.9 g/mol |
|---|---|
| Molecular Formula | C22H23ClFN5O3 |
| XLogP3 | 4.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 5 |
| Exact Mass | 459.1473455 g/mol |
| Monoisotopic Mass | 459.1473455 g/mol |
| Topological Polar Surface Area | 79.8 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 649 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
AZD3759 (Zorifertinib) is a small molecule drug, which is currently being evaluated in Phase I clinical studies for the treatment of carcinoma, non-small-cell lung.
Lead Product(s): Zorifertinib,Osimertinib Mesylate
Therapeutic Area: Oncology Brand Name: AZD3759
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 23, 2026

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Zorifertinib,Osimertinib Mesylate
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Zorifertinib With Osimertinib for NSCLC With Meningeal Progression
Details : AZD3759 (Zorifertinib) is a small molecule drug, which is currently being evaluated in Phase I clinical studies for the treatment of carcinoma, non-small-cell lung.
Product Name : AZD3759
Product Type : Miscellaneous
Upfront Cash : Inapplicable
January 23, 2026

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
Zorifer (zorifertinib HCl) indicated for the first-line treatment of adult patients with locally advanced or metastatic NSCLC accompanied with EGFR-L858R mutation
Lead Product(s): Zorifertinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Approved FDFProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable November 20, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Zorifertinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Approved FDF
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Alpha Biopharma Gets NMPA Approval for Zorifertinib for Lung Cancer Brain Metastases
Details : Zorifer (zorifertinib HCl) indicated for the first-line treatment of adult patients with locally advanced or metastatic NSCLC accompanied with EGFR-L858R mutation
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
November 20, 2024

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
AZD-3759 (zorifertinib) is a potent, oral, reversible, next-generation mutated EGFR-TKI with full blood-brain barrier penetration targeting advanced NSCLC patients with CNS metastases and EGFR-sensitizing mutations.
Lead Product(s): Zorifertinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase II/ Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable January 29, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Zorifertinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Phase II/ Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : AZD-3759 (zorifertinib) is a potent, oral, reversible, next-generation mutated EGFR-TKI with full blood-brain barrier penetration targeting advanced NSCLC patients with CNS metastases and EGFR-sensitizing mutations.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
January 29, 2023

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
The clinical and preclinical data of Zorifertinib have shown its high blood-brain barrier (BBB) penetration, anti-tumor activity in metastatic CNS lesions, overall efficacy in both CNS and extra-cranial diseases, and similar safety profile as other EGFR-TKI drugs. I
Lead Product(s): Zorifertinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase II/ Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Zorifertinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Phase II/ Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : The clinical and preclinical data of Zorifertinib have shown its high blood-brain barrier (BBB) penetration, anti-tumor activity in metastatic CNS lesions, overall efficacy in both CNS and extra-cranial diseases, and similar safety profile as other EGFR-...
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 24, 2022

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
AZD3759 is a Other Small Molecule drug candidate, which is currently being evaluated in phase II/ phase III clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Lead Product(s): Zorifertinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase II/ Phase IIIProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 31, 2018

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Zorifertinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Phase II/ Phase III
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : AZD3759 is a Other Small Molecule drug candidate, which is currently being evaluated in phase II/ phase III clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 31, 2018

Details:
Avitinib Maleate is a drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Lead Product(s):
Abivertinib,Afatinib,Chidamide,Crizotinib,Ensartinib,Pyrotinib Maleate,Zorifertinib,Pirotinib,Nimotuzumab,Gemcitabine,
Therapeutic Area: Oncology
Brand Name: Undisclosed
Study Phase: Phase IIProduct Type: Undisclosed Sponsor:
Chinese Thoracic Oncology Group
Deal Size: Inapplicable
Upfront Cash: Inapplicable
Deal Type: Inapplicable
July 02, 2018

Lead Product(s) : Abivertinib, Afatinib, Chidamide, Crizotinib, Ensartinib
Therapeutic Area : Oncology
Highest Development Status : Phase II
Partner/Sponsor/Collaborator : Chinese Thoracic Oncology Group
Deal Size : Inapplicable
Deal Type : Inapplicable
Phase II Umbrella Study Directed by Next Generation Sequencing
Details : Avitinib Maleate is a drug candidate, which is currently being evaluated in phase II clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Product Name : Undisclosed
Product Type : Undisclosed
Upfront Cash : Inapplicable
July 02, 2018

Details:
AZD3759 is a Other Small Molecule drug candidate, which is currently being evaluated in phase I/ phase II clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Lead Product(s): Zorifertinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase I/ Phase IIProduct Type: Miscellaneous
Sponsor: Tigermed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable December 04, 2017

Lead Product(s) : Zorifertinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Phase I/ Phase II
Partner/Sponsor/Collaborator : Tigermed
Deal Size : Inapplicable
Deal Type : Inapplicable
Evaluate Safety, Tolerability, Pharmacokinetics and Anti-tumor Activity of AZD3759
Details : AZD3759 is a Other Small Molecule drug candidate, which is currently being evaluated in phase I/ phase II clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
December 04, 2017

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Details:
AZD3759 is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Lead Product(s): Zorifertinib,Inapplicable
Therapeutic Area: Oncology Brand Name: Undisclosed
Study Phase: Phase IProduct Type: Miscellaneous
Sponsor: Undisclosed
Deal Size: Inapplicable Upfront Cash: Inapplicable
Deal Type: Inapplicable August 29, 2014

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Lead Product(s) : Zorifertinib,Inapplicable
Therapeutic Area : Oncology
Highest Development Status : Phase I
Partner/Sponsor/Collaborator : Undisclosed
Deal Size : Inapplicable
Deal Type : Inapplicable
Details : AZD3759 is a Other Small Molecule drug candidate, which is currently being evaluated in phase I clinical studies for the treatment of Carcinoma, Non-Small-Cell Lung.
Product Name : Undisclosed
Product Type : Miscellaneous
Upfront Cash : Inapplicable
August 29, 2014

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
24
PharmaCompass offers a list of Zorifertinib API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Zorifertinib manufacturer or Zorifertinib supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Zorifertinib manufacturer or Zorifertinib supplier.
PharmaCompass also assists you with knowing the Zorifertinib API Price utilized in the formulation of products. Zorifertinib API Price is not always fixed or binding as the Zorifertinib Price is obtained through a variety of data sources. The Zorifertinib Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Zorifertinib manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Zorifertinib, including repackagers and relabelers. The FDA regulates Zorifertinib manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Zorifertinib API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A Zorifertinib supplier is an individual or a company that provides Zorifertinib active pharmaceutical ingredient (API) or Zorifertinib finished formulations upon request. The Zorifertinib suppliers may include Zorifertinib API manufacturers, exporters, distributors and traders.
Zorifertinib Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Zorifertinib GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Zorifertinib GMP manufacturer or Zorifertinib GMP API supplier for your needs.
A Zorifertinib CoA (Certificate of Analysis) is a formal document that attests to Zorifertinib's compliance with Zorifertinib specifications and serves as a tool for batch-level quality control.
Zorifertinib CoA mostly includes findings from lab analyses of a specific batch. For each Zorifertinib CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Zorifertinib may be tested according to a variety of international standards, such as European Pharmacopoeia (Zorifertinib EP), Zorifertinib JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Zorifertinib USP).

