
Synopsis
Synopsis
0
CEP/COS
0
VMF
0
Australia

0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
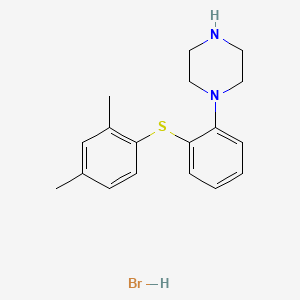

1. 1-(2-(2,4-dimethylphenylsulfanyl)phenyl)piperazine
2. Brintellix
3. Lu Aa21004
4. Lu-aa21004
5. Luaa21004
6. Vortioxetine
1. 960203-27-4
2. Vortioxetine Hbr
3. Brintellix
4. Vortioxetine (lu Aa21004) Hbr
5. Trintellix
6. Vortioxetine (hydrobromide)
7. Unii-tks641koay
8. Lu Aa 21004 Hydrobromide
9. Vortioxetine Hydrobromide [usan]
10. Lu Aa21004 Hbr
11. Tks641koay
12. Lu-aa21004 Hydrobromide
13. Lu Aa21004 (hbr)
14. 1-(2-((2,4-dimethylphenyl)thio)phenyl)piperazine Hydrobromide
15. 960203-27-4 (hbr)
16. Chebi:76015
17. 1-(2-((2,4-dimethylphenyl)sulfanyl)phenyl)piperazine Monohydrobromide
18. Brintellix (tn)
19. 1-[2-(2,4-dimethylphenyl)sulfanylphenyl]piperazine Hydrobromide
20. 1-[2-[(2,4-dimethylphenyl)thio]phenyl]piperazine Hydrobromide
21. Piperazine, 1-(2-((2,4-dimethylphenyl)thio)phenyl)-, Hydrobromide (1:1)
22. Trintellix (tn)
23. Voltiocetin Hydrobromide
24. Lu Aa21004 Hydrobromide
25. Vortioxetine Monohydrobromide
26. Schembl237653
27. Lu-aa21004 Hbr
28. Chembl2107387
29. Lu-aa-21004
30. Dtxsid501027850
31. Amy25254
32. Bcp09588
33. Ex-a2348
34. Hy-15414a
35. Mfcd22383961
36. S8021
37. Vortioxetine Hydrobromide (jan/usan)
38. Akos016340374
39. Ccg-268394
40. Cs-1472
41. Hg-0011
42. Sb16507
43. Vortioxetine Hydrobromide [jan]
44. Ac-28325
45. Vortioxetine (lu Aa21004) Hydrobromide
46. Vortioxetine Hydrobromide [who-dd]
47. 960203-27-4, Trintellix, Brintellix,
48. Ft-0696676
49. Sw219360-1
50. D10185
51. Vortioxetine Hydrobromide [orange Book]
52. A855301
53. Lu Aa 21004 Hydrobromide;lu Aa21004 Hydrobromide
54. Q27145682
55. 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine-hbr
56. 1-[2-(2,4-dimethylphenyl)sulfanylphenyl]piperazine;hydrobromide
57. 1-[2-[(2,4-dimethylphenyl)thio]phenyl]-piperazine Hydrobromide
58. 1-{2-[(2,4-dimethylphenyl)sulfanyl]phenyl}piperazine Hydrobromide
59. 4-[2-(2,4-dimethyl-phenylsulfanyl)-phenyl]-piperazine Hydrobromide
60. 4-{2-[(2,4-dimethylphenyl)sulfanyl]phenyl}piperazin-1-ium Bromide
1. Vortioxetine
| Molecular Weight | 379.4 g/mol |
|---|---|
| Molecular Formula | C18H23BrN2S |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 3 |
| Exact Mass | 378.07653 g/mol |
| Monoisotopic Mass | 378.07653 g/mol |
| Topological Polar Surface Area | 40.6 Ų |
| Heavy Atom Count | 22 |
| Formal Charge | 0 |
| Complexity | 316 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Brintellix |
| PubMed Health | Meclizine, Buclizine, and Cyclizine (Oral route, Parenteral route) |
| Drug Label | BRINTELLIX is an immediate-release tablet for oral administration that contains the beta () polymorph of vortioxetine hydrobromide (HBr), an antidepreant. Vortioxetine HBr is known chemically as 1-[2-(2,4-Dimethyl-phenylsulfanyl)-phenyl]-piperazi... |
| Active Ingredient | Vortioxetine hydrobromide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 15mg base; eq 5mg base; eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
| 2 of 2 | |
|---|---|
| Drug Name | Brintellix |
| PubMed Health | Meclizine, Buclizine, and Cyclizine (Oral route, Parenteral route) |
| Drug Label | BRINTELLIX is an immediate-release tablet for oral administration that contains the beta () polymorph of vortioxetine hydrobromide (HBr), an antidepreant. Vortioxetine HBr is known chemically as 1-[2-(2,4-Dimethyl-phenylsulfanyl)-phenyl]-piperazi... |
| Active Ingredient | Vortioxetine hydrobromide |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 15mg base; eq 5mg base; eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Takeda Pharms Usa |
Treatment of major depressive episodes in adults.
Anti-Anxiety Agents
Agents that alleviate ANXIETY, tension, and ANXIETY DISORDERS, promote sedation, and have a calming effect without affecting clarity of consciousness or neurologic conditions. ADRENERGIC BETA-ANTAGONISTS are commonly used in the symptomatic treatment of anxiety but are not included here. (See all compounds classified as Anti-Anxiety Agents.)
Antidepressive Agents
Mood-stimulating drugs used primarily in the treatment of affective disorders and related conditions. Several MONOAMINE OXIDASE INHIBITORS are useful as antidepressants apparently as a long-term consequence of their modulation of catecholamine levels. The tricyclic compounds useful as antidepressive agents (ANTIDEPRESSIVE AGENTS, TRICYCLIC) also appear to act through brain catecholamine systems. A third group (ANTIDEPRESSIVE AGENTS, SECOND-GENERATION) is a diverse group of drugs including some that act specifically on serotonergic systems. (See all compounds classified as Antidepressive Agents.)
Selective Serotonin Reuptake Inhibitors
Compounds that specifically inhibit the reuptake of serotonin in the brain. (See all compounds classified as Selective Serotonin Reuptake Inhibitors.)
Serotonin 5-HT1 Receptor Agonists
Endogenous compounds and drugs that specifically stimulate SEROTONIN 5-HT1 RECEPTORS. Included under this heading are agonists for one or more of the specific 5-HT1 receptor subtypes. (See all compounds classified as Serotonin 5-HT1 Receptor Agonists.)
Serotonin 5-HT3 Receptor Antagonists
Drugs that bind to but do not activate SEROTONIN 5-HT3 RECEPTORS, thereby blocking the actions of SEROTONIN or SEROTONIN 5-HT3 RECEPTOR AGONISTS. (See all compounds classified as Serotonin 5-HT3 Receptor Antagonists.)
N06AX26
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
 Click Us!
Click Us! 
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 41091
Submission : 2024-12-31
Status : Active
Type : II

NDC Package Code : 42765-070
Start Marketing Date : 2024-12-31
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (15kg/15kg)
Marketing Category : BULK INGREDIENT
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.

GDUFA
DMF Review : Reviewed
Rev. Date : 2017-04-14
Pay. Date : 2017-03-03
DMF Number : 31314
Submission : 2017-03-01
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 34873
Submission : 2020-05-14
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 38974
Submission : 2023-10-10
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2017-04-18
Pay. Date : 2017-03-28
DMF Number : 31602
Submission : 2017-03-30
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 35801
Submission : 2021-03-31
Status : Active
Type : II
Date of Issue : 2022-06-29
Valid Till : 2025-02-07
Written Confirmation Number : WC-0123
Address of the Firm :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
 Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 41091
Submission : 2024-12-31
Status : Active
Type : II
GDUFA
DMF Review : Complete
Rev. Date : 2017-02-01
Pay. Date : 2016-12-27
DMF Number : 31281
Submission : 2016-12-30
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 29849
Submission : 2016-08-15
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-04-13
Pay. Date : 2017-02-23
DMF Number : 31389
Submission : 2017-03-04
Status : Active
Type : II

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 21224
Submission : 2008-01-17
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-04-14
Pay. Date : 2017-03-03
DMF Number : 31314
Submission : 2017-03-01
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-05-17
Pay. Date : 2017-03-16
DMF Number : 31507
Submission : 2017-03-23
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-07-31
Pay. Date : 2017-05-11
DMF Number : 31411
Submission : 2017-02-28
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-02-03
Pay. Date : 2016-09-29
DMF Number : 30309
Submission : 2016-03-10
Status : Active
Type : II

GDUFA
DMF Review : Complete
Rev. Date : 2017-05-24
Pay. Date : 2017-03-27
DMF Number : 31583
Submission : 2017-03-31
Status : Active
Type : II

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Registration Number : 230MF10077
Registrant's Address : Ottiliavej 9, DK-2500 Valby, Copenhagen, Denmark
Initial Date of Registration : 2018-07-03
Latest Date of Registration : 2021-11-02

Registration Number : 230MF10076
Registrant's Address : Via Quarta Strada, 2 Padova, Italy
Initial Date of Registration : 2018-07-03
Latest Date of Registration : 2021-11-02

Registration Number : 304MF10041
Registrant's Address : 7-2-A2,Hetero Corporate,Industrial Estates Sanath Nagar,Hyderabad-500 018 Telangana I...
Initial Date of Registration : 2022-02-24
Latest Date of Registration : 2022-02-24

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
Metrochem has been delivering customized volume & quality products to customers across the world, taking utmost care of their needs.
About the Company : Established in 2004, Metrochem API is one of the fastest-growing APIs, pellets & intermediates manufacturers. It has 6 dedicated manufacturing facilities for its 3 core product gro...
 LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
LGM Pharma accelerates & optimizes the new product pathway from early development through commercialization.
About the Company : LGM Pharma is a global leader in sourcing hard-to-find APIs and intermediates for the pharmaceutical and biotech industries. LGM is also a full service CDMO providing formulation, ...
About the Company : HRV Global is a leading global manufacturer, seller & exporter of a wide range of APIs, advanced intermediates, pellets, food grade chemicals, food additives & food ingredients. It...
About the Company : Established in May 2012, Shandong Loncom Pharmaceutical operates as a fully owned subsidiary of Shandong Bestcomm Pharmaceutical Co., Ltd. Situated in the Qihe Economic Development...
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
About the Company : Tenatra International was established as a proprietorship firm in 1999. It got off to a very good start, supporting clients in the United States, Mexico and Europe. As business opp...
About the Company : Beijing Hope Pharmaceutical Co., Ltd., established in 2005, is a high-tech enterprise with "chemical drug research and development" as its core capability. Hope has over 16 years o...

About the Company : Centaur accepts change as a constant, and continuously innovates to remain significant. Centaur has built knowledge sharing relationships with the pharmaceutical majors in areas of...

About the Company : MANA PHARMA, S.L. is a Spanish Pharmaceutical Company, with Authorization as a Wholesale Pharmaceutical Distribution Entity of Medicines for Human Use as well as a Certificate of G...

About the Company : Established in 1984, R L Fine Chem Pvt. Ltd. is one of the fastest growing API companies, with a leadership position in several APIs such as antihistamines, antidepressants and mus...

About the Company : Raghava Life Sciences Pvt. Ltd. (RLS), established in the year 2018, is an integrated small molecules chemistry Contract research, Development and Manufacturing Organization (CDMO)...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Vortioxetine 5 mg Aspen
Dosage Form : TAB
Dosage Strength : 5mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Vortioxetine 10 mg Aspen
Dosage Form : TAB
Dosage Strength : 10mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Vortioxetine 20 mg Aspen
Dosage Form : TAB
Dosage Strength : 20mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa
 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Canada
VORTIOXETINE (VORTIOXETINE HYDROBROMIDE)
Brand Name : APO-VORTIOXETINE
Dosage Form : TABLET
Dosage Strength : 5MG
Packaging :
Approval Date :
Application Number : 2552744
Regulatory Info :
Registration Country : Canada

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Vorellix 5
Dosage Form : FCT
Dosage Strength : 5mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Brintellix
Dosage Form : Vortioxetina 20Mg/Ml 15Ml Oral Use
Dosage Strength : os gtt 15 ml 20 mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Brintellix
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 5 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : Generic
Registration Country : South Africa
Brand Name : Brivor 5 mg
Dosage Form : FCT
Dosage Strength : 5mg
Packaging : 30X1mg
Approval Date :
Application Number :
Regulatory Info : Generic
Registration Country : South Africa

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : USA
Brand Name : VORTIOXETINE
Dosage Form : TABLET;ORAL
Dosage Strength : 20MG
Packaging :
Approval Date :
Application Number : 211024
Regulatory Info :
Registration Country : USA

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info : DISCN
Registration Country : USA
Brand Name : TRINTELLIX
Dosage Form : TABLET;ORAL
Dosage Strength : EQ 15MG BASE **Federal Register determination that product was not discontinued or withdrawn for safety or effectiveness reasons**
Packaging :
Approval Date : 2013-09-30
Application Number : 204447
Regulatory Info : DISCN
Registration Country : USA

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results] Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
vortioxetinhydrobromid, anhydrous
Brand Name : Brintellix
Dosage Form : FILM COATED PILL
Dosage Strength : 20 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Sweden
vortioxetinhydrobromid, anhydrous
Brand Name : Brintellix
Dosage Form : FILM COATED PILL
Dosage Strength : 5 MG
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Sweden

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Italy
Brand Name : Brintellix
Dosage Form : Vortioxetina 10Mg 28 Combined Oral Use
Dosage Strength : 28 cpr riv 10 mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Italy

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Brintellix
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 10 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Brintellix
Dosage Form : Antic-calc Tablet, Film Coated
Dosage Strength : 20 mg
Packaging : Blister
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Norway
Brand Name : Brintellix
Dosage Form : Tablet, film-coated
Dosage Strength : 10 mg
Packaging : Blisterpakning 98item
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Norway

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Brintellix
Dosage Form : Filmtable
Dosage Strength : 10mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Brintellix
Dosage Form : Filmtable
Dosage Strength : 20mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Brintellix
Dosage Form : Tropfen
Dosage Strength : 20mg/ml
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Boost your online visibility by uploading your products, APIs, FDFs, intermediates, excipients, and services for free on PharmaCompass.
Rank higher among suppliers and expand your reach across the internet efficiently and cost-effectively.
Regulatory Info :
Registration Country : Switzerland
Brand Name : Brintellix
Dosage Form : Filmtable
Dosage Strength : 20mg
Packaging :
Approval Date :
Application Number :
Regulatory Info :
Registration Country : Switzerland

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]DRUG PRODUCT COMPOSITIONS

Related Excipient Companies

Dosage Form : Tablet
Grade : Oral
Application : Taste Masking
Excipient Details : Mannitol is used as a filler, bulking agent and taste masking agent in ODT formulations such as tablets.
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Application : Disintegrants & Superdisintegrants
Excipient Details : Mannogem 2080 exhibits excellent flow, disintegration and compression properties.
Dosage Form : Orodispersible Tablet
Grade : Oral
Dosage Form : Orodispersible Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
Dosage Form : Tablet
Grade : Oral
 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
Patent Expiration Date : 2027-06-15
US Patent Number : 8969355
Drug Substance Claim :
Drug Product Claim :
Application Number : 204447
Patent Use Code : U-1668
Delist Requested :
Patent Use Description : METHOD OF TREATING DEP...
Patent Expiration Date : 2027-06-15

Patent Expiration Date : 2027-12-15
US Patent Number : 9125910*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 204447
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2027-12-15

Patent Expiration Date : 2027-12-15
US Patent Number : 9861630*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 204447
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2027-12-15

Patent Expiration Date : 2027-06-15
US Patent Number : 8969355
Drug Substance Claim :
Drug Product Claim :
Application Number : 204447
Patent Use Code : U-1668
Delist Requested :
Patent Use Description : METHOD OF TREATING DEP...
Patent Expiration Date : 2027-06-15

Patent Expiration Date : 2031-06-30
US Patent Number : 8722684
Drug Substance Claim : Y
Drug Product Claim : Y
Application Number : 204447
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2031-06-30

Patent Expiration Date : 2027-06-15
US Patent Number : 9861630
Drug Substance Claim :
Drug Product Claim :
Application Number : 204447
Patent Use Code : U-1668
Delist Requested :
Patent Use Description : METHOD OF TREATING DEP...
Patent Expiration Date : 2027-06-15

Patent Expiration Date : 2027-06-15
US Patent Number : 9125908
Drug Substance Claim :
Drug Product Claim :
Application Number : 204447
Patent Use Code : U-2309
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2027-06-15

Patent Expiration Date : 2027-12-15
US Patent Number : 9125908*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 204447
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2027-12-15

Patent Expiration Date : 2027-06-15
US Patent Number : 9125910
Drug Substance Claim :
Drug Product Claim :
Application Number : 204447
Patent Use Code : U-2309
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2027-06-15

Patent Expiration Date : 2027-12-15
US Patent Number : 9125909*PED
Drug Substance Claim :
Drug Product Claim :
Application Number : 204447
Patent Use Code :
Delist Requested :
Patent Use Description :
Patent Expiration Date : 2027-12-15

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]REF. STANDARDS & IMPURITIES

CAS Number : 960203-27-4
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : V0026.62

CAS Number : 13616-83-6
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : V0026.18

CAS Number : 960203-27-4 (free base)
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : V0026.07

CAS Number :
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : V0026.35

CAS Number : 1013-76-9
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : V0026.39

CAS Number : 108-86-1
Quantity Per Vial :
Sale Unit :
Price :
Details : In stock
Monograph :
Storage :
Code/Batch No : V0026.55

N-acetyl vortioxetine IMPURITY
CAS Number : 1801352-86-2
Quantity Per Vial :
Sale Unit :
Price :
Details : Cat No. CS-O-16869 // Impurity
Monograph :
Storage :
Code/Batch No :

CAS Number : 2925447-38-5
Quantity Per Vial :
Sale Unit :
Price :
Details :
Monograph :
Storage :
Code/Batch No :

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]ABOUT THIS PAGE
19
PharmaCompass offers a list of Vortioxetine Hydrobromide API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vortioxetine Hydrobromide manufacturer or Vortioxetine Hydrobromide supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vortioxetine Hydrobromide manufacturer or Vortioxetine Hydrobromide supplier.
PharmaCompass also assists you with knowing the Vortioxetine Hydrobromide API Price utilized in the formulation of products. Vortioxetine Hydrobromide API Price is not always fixed or binding as the Vortioxetine Hydrobromide Price is obtained through a variety of data sources. The Vortioxetine Hydrobromide Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A vortioxetine monohydrobromide manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of vortioxetine monohydrobromide, including repackagers and relabelers. The FDA regulates vortioxetine monohydrobromide manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. vortioxetine monohydrobromide API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of vortioxetine monohydrobromide manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A vortioxetine monohydrobromide supplier is an individual or a company that provides vortioxetine monohydrobromide active pharmaceutical ingredient (API) or vortioxetine monohydrobromide finished formulations upon request. The vortioxetine monohydrobromide suppliers may include vortioxetine monohydrobromide API manufacturers, exporters, distributors and traders.
click here to find a list of vortioxetine monohydrobromide suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A vortioxetine monohydrobromide DMF (Drug Master File) is a document detailing the whole manufacturing process of vortioxetine monohydrobromide active pharmaceutical ingredient (API) in detail. Different forms of vortioxetine monohydrobromide DMFs exist exist since differing nations have different regulations, such as vortioxetine monohydrobromide USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A vortioxetine monohydrobromide DMF submitted to regulatory agencies in the US is known as a USDMF. vortioxetine monohydrobromide USDMF includes data on vortioxetine monohydrobromide's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The vortioxetine monohydrobromide USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of vortioxetine monohydrobromide suppliers with USDMF on PharmaCompass.
The Pharmaceuticals and Medical Devices Agency (PMDA) established the Japan Drug Master File (JDMF), also known as the Master File (MF), to permit Japanese and foreign manufacturers of drug substances, intermediates, excipients, raw materials, and packaging materials (‘Products’) to voluntarily register confidential information about the production and management of their products in Japan.
The vortioxetine monohydrobromide Drug Master File in Japan (vortioxetine monohydrobromide JDMF) empowers vortioxetine monohydrobromide API manufacturers to present comprehensive information (e.g., production methods, data, etc.) to the review authority, i.e., PMDA (Pharmaceuticals & Medical Devices Agency).
PMDA reviews the vortioxetine monohydrobromide JDMF during the approval evaluation for pharmaceutical products. At the time of vortioxetine monohydrobromide JDMF registration, PMDA checks if the format is accurate, if the necessary items have been included (application), and if data has been attached.
click here to find a list of vortioxetine monohydrobromide suppliers with JDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a vortioxetine monohydrobromide Drug Master File in Korea (vortioxetine monohydrobromide KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of vortioxetine monohydrobromide. The MFDS reviews the vortioxetine monohydrobromide KDMF as part of the drug registration process and uses the information provided in the vortioxetine monohydrobromide KDMF to evaluate the safety and efficacy of the drug.
After submitting a vortioxetine monohydrobromide KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their vortioxetine monohydrobromide API can apply through the Korea Drug Master File (KDMF).
click here to find a list of vortioxetine monohydrobromide suppliers with KDMF on PharmaCompass.
A vortioxetine monohydrobromide written confirmation (vortioxetine monohydrobromide WC) is an official document issued by a regulatory agency to a vortioxetine monohydrobromide manufacturer, verifying that the manufacturing facility of a vortioxetine monohydrobromide active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting vortioxetine monohydrobromide APIs or vortioxetine monohydrobromide finished pharmaceutical products to another nation, regulatory agencies frequently require a vortioxetine monohydrobromide WC (written confirmation) as part of the regulatory process.
click here to find a list of vortioxetine monohydrobromide suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing vortioxetine monohydrobromide as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for vortioxetine monohydrobromide API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture vortioxetine monohydrobromide as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain vortioxetine monohydrobromide and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a vortioxetine monohydrobromide NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of vortioxetine monohydrobromide suppliers with NDC on PharmaCompass.
vortioxetine monohydrobromide Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of vortioxetine monohydrobromide GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right vortioxetine monohydrobromide GMP manufacturer or vortioxetine monohydrobromide GMP API supplier for your needs.
A vortioxetine monohydrobromide CoA (Certificate of Analysis) is a formal document that attests to vortioxetine monohydrobromide's compliance with vortioxetine monohydrobromide specifications and serves as a tool for batch-level quality control.
vortioxetine monohydrobromide CoA mostly includes findings from lab analyses of a specific batch. For each vortioxetine monohydrobromide CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
vortioxetine monohydrobromide may be tested according to a variety of international standards, such as European Pharmacopoeia (vortioxetine monohydrobromide EP), vortioxetine monohydrobromide JP (Japanese Pharmacopeia) and the US Pharmacopoeia (vortioxetine monohydrobromide USP).
