
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
VMF
0
Canada
DRUG PRODUCT COMPOSITIONS
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
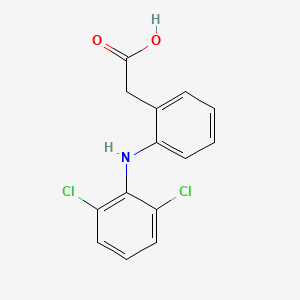

1. Dichlofenal
2. Diclofenac Potassium
3. Diclofenac Sodium
4. Diclofenac, Sodium
5. Diclonate P
6. Diclophenac
7. Dicrofenac
8. Feloran
9. Gp 45,840
10. Gp-45,840
11. Gp45,840
12. Novapirina
13. Orthofen
14. Orthophen
15. Ortofen
16. Sodium Diclofenac
17. Sr 38
18. Sr-38
19. Sr38
20. Voltaren
21. Voltarol
1. 15307-86-5
2. Diclofenac Acid
3. Dichlofenac
4. Diclofenacum
5. Diclophenac
6. 2-(2-((2,6-dichlorophenyl)amino)phenyl)acetic Acid
7. Diclofenaco
8. Diclofenac Resinate
9. Voltarol
10. 2-(2,6-dichloroanilino)phenylacetic Acid
11. 2-[2-(2,6-dichloroanilino)phenyl]acetic Acid
12. Benzeneacetic Acid, 2-[(2,6-dichlorophenyl)amino]-
13. Diclofenac Free Acid
14. 2-{2-[(2,6-dichlorophenyl)amino]phenyl}acetic Acid
15. 2-((2,6-dichlorophenyl)amino)benzeneacetic Acid
16. Solaraze
17. {2-[(2,6-dichlorophenyl)amino]phenyl}acetic Acid
18. Chebi:47381
19. Voltaren-xr
20. Benzeneacetic Acid, 2-((2,6-dichlorophenyl)amino)-
21. [2-(2,6-dichloroanilino)phenyl]acetic Acid
22. 2-[(2,6-dichlorophenyl)amino]benzeneacetic Acid
23. Chembl139
24. 2-[2,6-dichlorophenyl)amino]benzeneacetic Acid
25. Acetic Acid, (o-(2,6-dichloroanilino)phenyl)-
26. 144o8ql0l1
27. Las41007
28. Arthrotec
29. 15307-86-5 (free)
30. Prosorb-d
31. Olfen
32. Las-41007
33. Mfcd00056694
34. Solaraze (tn)
35. 2-[(2,6-dichlorophenyl)amino]-benzeneacetic Acid
36. Enfenamic Acid; N-phenethylanthranilic Acid; Rh 8
37. 2-[2-[(2,6-dichlorophenyl)amino]phenyl]acetic Acid
38. Zorovolex
39. Zorvolex
40. 2-[2-[(2,6-dichlorophenyl)amino]phenyl]acetic Acid (diclofenac)
41. Diclofenamic Acid
42. Diclofenacum [inn-latin]
43. Diclofenaco [inn-spanish]
44. Dif
45. Hsdb 7234
46. Einecs 239-348-5
47. (2-((2,6-dichlorophenyl)amino-phenyl)acetic Acid (hd)
48. Brn 2146636
49. (2-((2,6-dichlorophenyl)amino)phenyl)acetic Acid
50. Diclofenac [usan:inn:ban]
51. Unii-144o8ql0l1
52. Isv-205
53. Zorvolex (tn)
54. Spectrum_000930
55. Diclofenac [mi]
56. Diclofenac [inn]
57. 128402-48-2
58. Diclofenac (usan/inn)
59. Prestwick0_000594
60. Prestwick1_000594
61. Prestwick2_000594
62. Prestwick3_000594
63. Spectrum2_000991
64. Spectrum3_000385
65. Spectrum4_000506
66. Spectrum5_000867
67. Diclofenac [hsdb]
68. Diclofenac [usan]
69. Diclofenac [vandf]
70. Epitope Id:116873
71. Ec 239-348-5
72. Diclofenac [mart.]
73. Schembl2799
74. Diclofenac [who-dd]
75. Lopac0_000441
76. Oprea1_011155
77. Bspbio_000468
78. Bspbio_002169
79. Kbiogr_001051
80. Kbiogr_002306
81. Kbioss_001410
82. Kbioss_002308
83. Mls006011795
84. Bidd:gt0380
85. Divk1c_000272
86. Divk1c_000402
87. 2-[2-(2,6-dichlorophenyl)aminophenyl]ethanoic Acid
88. Spbio_001081
89. Spbio_002687
90. Bpbio1_000516
91. Gtpl2714
92. Zinc1281
93. Diclofenac [orange Book]
94. Dtxsid6022923
95. Bdbm13066
96. Hms501e04
97. Kbio1_000272
98. Kbio1_000402
99. Kbio2_001410
100. Kbio2_002306
101. Kbio2_003978
102. Kbio2_004874
103. Kbio2_006546
104. Kbio2_007442
105. Kbio3_001389
106. Kbio3_002786
107. 2b17
108. Cmap_000014
109. Ninds_000272
110. Ninds_000402
111. Hms2090c10
112. Hms3886f09
113. Bcp09087
114. Bcp13860
115. S6073
116. Stk984493
117. Akos001579542
118. Db00586
119. Ks-1258
120. Idi1_000272
121. Idi1_000402
122. Ncgc00021125-01
123. Ncgc00021125-02
124. Ncgc00021125-11
125. Ac-27673
126. Hy-15036
127. O-(2,6-dichloroanilino)phenylacetic Acid
128. Smr001550371
129. Sy038623
130. Sbi-0051341.p003
131. Diclofenac 1000 Microg/ml In Acetonitrile
132. Aceclofenac Impurity A [ep Impurity]
133. D3748
134. Ft-0624731
135. Ft-0666643
136. Unm000001216103
137. [2-(2,6-dichloroanilino)phenyl]acetic Acid #
138. C01690
139. D07816
140. H10425
141. [o-(2,6-dichloro-anilino)-phenyl]-acetic Acid
142. 2-[(2,6-dichlorophenyl)amino]phenylacetic Acid
143. 2-[2-(2,6-dichloroanilino)phenyl]acetic Acid.
144. Ab01275502-01
145. Ab01275502_02
146. 056d694
147. 2-(2-(2,6-dichlorophenylamino)phenyl)acetic Acid
148. 2-[(2,6-dichlorophenyl)amino]-phenyl-acetic Acid
149. Q244408
150. (o-(2,6-dichloroanilino)phenyl)acetic Acid
151. [2-(2,6-dichloro-phenylamino)-phenyl]-acetic Acid
152. 2-[(2,6-dichlorophenyl)-amino]-benzeneacetic Acid
153. 2-[(2,6-dichlorophenyl)-amino]-phenyl-acetic Acid
154. 2-[2-(2,6-dichlorophenylamino)phenyl]-acetic Acid
155. Sr-01000003041-3
156. 2-[2-(2,6-dichlorophenylamino)-phenyl]-acetic Acid
157. Brd-k08252256-236-05-6
158. Brd-k08252256-236-17-1
159. Z57664869
160. F0722-0745
| Molecular Weight | 296.1 g/mol |
|---|---|
| Molecular Formula | C14H11Cl2NO2 |
| XLogP3 | 4.4 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 295.0166840 g/mol |
| Monoisotopic Mass | 295.0166840 g/mol |
| Topological Polar Surface Area | 49.3 Ų |
| Heavy Atom Count | 19 |
| Formal Charge | 0 |
| Complexity | 304 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 10 | |
|---|---|
| Drug Name | Cataflam |
| PubMed Health | Diclofenac |
| Drug Classes | Analgesic, Anti-Inflammatory, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, Ophthalmologic Agent |
| Drug Label | Cataflam (diclofenac potassium immediate-release tablets) is a benzeneacetic acid derivative. Cataflam is available as immediate-release tablets of 50 mg (light brown) for oral administration. The chemical name is 2-[(2,6-dichlorophenyl)amino] benz... |
| Active Ingredient | Diclofenac potassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Novartis |
| 2 of 10 | |
|---|---|
| Drug Name | Pennsaid |
| PubMed Health | Diclofenac |
| Drug Classes | Analgesic, Anti-Inflammatory, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, Ophthalmologic Agent |
| Drug Label | PENNSAID is a clear, colorless to faintly pink-orange solution for topical application.PENNSAID contains 1.5% w/w diclofenac sodium, a benzeneacetic acid derivative that is a nonsteroidal anti-inflammatory drug (NSAID), designated chemically as 2-[(2... |
| Active Ingredient | Diclofenac sodium |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 1.5%; 2% |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 3 of 10 | |
|---|---|
| Drug Name | Solaraze |
| PubMed Health | Diclofenac |
| Drug Classes | Analgesic, Anti-Inflammatory, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, Ophthalmologic Agent |
| Drug Label | Solaraze (diclofenac sodium) Gel, 3%, contains the active ingredient, diclofenac sodium, in a clear, transparent, colorless to slightly yellow gel base. Diclofenac sodium is a white to slightly yellow crystalline powder. It is freely soluble in met... |
| Active Ingredient | Diclofenac sodium |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 3% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 4 of 10 | |
|---|---|
| Drug Name | Voltaren |
| PubMed Health | Diclofenac |
| Drug Classes | Analgesic, Anti-Inflammatory, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, Ophthalmologic Agent |
| Drug Label | Voltaren Gel (diclofenac sodium topical gel) is a nonsteroidal anti-inflammatory drug (NSAID) for topicaluse only. It contains the active ingredient, diclofenac sodium, in an opaque, white gel base. Diclofenacsodium is a white to slightly yello... |
| Active Ingredient | Diclofenac sodium |
| Dosage Form | Solution/drops; Gel |
| Route | Ophthalmic; Topical |
| Strength | 1%; 0.1% |
| Market Status | Prescription |
| Company | Novartis |
| 5 of 10 | |
|---|---|
| Drug Name | Voltaren-xr |
| PubMed Health | Diclofenac (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Voltaren-XR (diclofenac sodium extended-release) tablets, USPis a benzeneacetic acid derivative. Voltaren-XR is available as extended-release tablets of 100 mg (light pink) for oral administration. The chemical name is 2-[(2,6-dichlorophenyl)amin... |
| Active Ingredient | Diclofenac sodium |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Novartis |
| 6 of 10 | |
|---|---|
| Drug Name | Cataflam |
| PubMed Health | Diclofenac |
| Drug Classes | Analgesic, Anti-Inflammatory, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, Ophthalmologic Agent |
| Drug Label | Cataflam (diclofenac potassium immediate-release tablets) is a benzeneacetic acid derivative. Cataflam is available as immediate-release tablets of 50 mg (light brown) for oral administration. The chemical name is 2-[(2,6-dichlorophenyl)amino] benz... |
| Active Ingredient | Diclofenac potassium |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Novartis |
| 7 of 10 | |
|---|---|
| Drug Name | Pennsaid |
| PubMed Health | Diclofenac |
| Drug Classes | Analgesic, Anti-Inflammatory, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, Ophthalmologic Agent |
| Drug Label | PENNSAID is a clear, colorless to faintly pink-orange solution for topical application.PENNSAID contains 1.5% w/w diclofenac sodium, a benzeneacetic acid derivative that is a nonsteroidal anti-inflammatory drug (NSAID), designated chemically as 2-[(2... |
| Active Ingredient | Diclofenac sodium |
| Dosage Form | Solution |
| Route | Topical |
| Strength | 1.5%; 2% |
| Market Status | Prescription |
| Company | Mallinckrodt |
| 8 of 10 | |
|---|---|
| Drug Name | Solaraze |
| PubMed Health | Diclofenac |
| Drug Classes | Analgesic, Anti-Inflammatory, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, Ophthalmologic Agent |
| Drug Label | Solaraze (diclofenac sodium) Gel, 3%, contains the active ingredient, diclofenac sodium, in a clear, transparent, colorless to slightly yellow gel base. Diclofenac sodium is a white to slightly yellow crystalline powder. It is freely soluble in met... |
| Active Ingredient | Diclofenac sodium |
| Dosage Form | Gel |
| Route | Topical |
| Strength | 3% |
| Market Status | Prescription |
| Company | Fougera Pharms |
| 9 of 10 | |
|---|---|
| Drug Name | Voltaren |
| PubMed Health | Diclofenac |
| Drug Classes | Analgesic, Anti-Inflammatory, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent, Ophthalmologic Agent |
| Drug Label | Voltaren Gel (diclofenac sodium topical gel) is a nonsteroidal anti-inflammatory drug (NSAID) for topicaluse only. It contains the active ingredient, diclofenac sodium, in an opaque, white gel base. Diclofenacsodium is a white to slightly yello... |
| Active Ingredient | Diclofenac sodium |
| Dosage Form | Solution/drops; Gel |
| Route | Ophthalmic; Topical |
| Strength | 1%; 0.1% |
| Market Status | Prescription |
| Company | Novartis |
| 10 of 10 | |
|---|---|
| Drug Name | Voltaren-xr |
| PubMed Health | Diclofenac (By mouth) |
| Drug Classes | Analgesic, Antimigraine, Antirheumatic, Central Nervous System Agent, Musculoskeletal Agent |
| Drug Label | Voltaren-XR (diclofenac sodium extended-release) tablets, USPis a benzeneacetic acid derivative. Voltaren-XR is available as extended-release tablets of 100 mg (light pink) for oral administration. The chemical name is 2-[(2,6-dichlorophenyl)amin... |
| Active Ingredient | Diclofenac sodium |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 100mg |
| Market Status | Prescription |
| Company | Novartis |
Anti-Inflammatory Agents, Non-Steroidal; Cyclooxygenase Inhibitors
National Library of Medicine's Medical Subject Headings online file (MeSH, 2011)
Diclofenac sodium also is used topically as an ophthalmic solution for the treatment of postoperative ocular inflammation in patients undergoing cataract extraction. /Diclofenac sodium; Included in US product labeling/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2108
Oral diclofenac sodium has been used for its antipyretic effect in the management of fever, usually associated with infection. In one study, the antipyretic effect of usual dosages of diclofenac sodium as delayed-release (enteric-coated) tablets was about equal to that of usual dosages of aspirin. The drug, however, should not be used routinely as an antipyretic because of its potential adverse effects. /Diclofenac sodium; NOT included in US product labeling/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2108
Diclofenac sodium as delayed-release (enteric-coated) tablets also has been used for the symptomatic relief of dysmenorrhea. /Diclofenac sodium; NOT included in US product labeling/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2108
For more Therapeutic Uses (Complete) data for DICLOFENAC (22 total), please visit the HSDB record page.
Pregnancy risk category: B /NO EVIDENCE OF RISK IN HUMANS. Adequate, well controlled studies in pregnant women have not shown increased risk of fetal abnormalities despite adverse findings in animals, or, in the absence of adequate human studies, animal studies show no fetal risk. The chance of fetal harm is remote but remains a possibility./
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 3567
Not recommended for patients with blood dyscrasias (or history of) or bone marrow depression.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 390
Diclofenac sodium in fixed combination with misoprostol is contraindicated in women who are pregnant because misoprostol exhibits abortifacient activity and can cause serious fetal harm. In addition, it is recommended that diclofenac in fixed combination with misoprostol be used in women of childbearing potential only if they require nonsteroidal anti-inflammatory agent (NSAIA) therapy and are considered at high risk of complications resulting from NSAIA-induced gastric or duodenal ulceration or at high risk of developing gastric or duodenal ulceration. /Diclofenac sodium/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2111
Caution with diclofenac sodium-containing dosage forms in patients who must restrict their sodium intake.
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 390
For more Drug Warnings (Complete) data for DICLOFENAC (24 total), please visit the HSDB record page.
Diclofenac is indicated for use in the treatment of pain and inflammation from varying sources including inflammatory conditions such as osteoarthritis, rheumatoid arthritis, and akylosing spondylitis, as well as injury-related inflammation due to surgery and physical trauma. It is often used in combination with [misoprostol] as a gastro-protective agent in patients with high risk of developing NSAID-induced ulcers.
FDA Label
Diclofenac reduces inflammation and by extension reduces nociceptive pain and combats fever. It also increases the risk of developing a gastrointestinal ulcer by inhibiting the production of protective mucus in the stomach.
Anti-Inflammatory Agents, Non-Steroidal
Anti-inflammatory agents that are non-steroidal in nature. In addition to anti-inflammatory actions, they have analgesic, antipyretic, and platelet-inhibitory actions. They act by blocking the synthesis of prostaglandins by inhibiting cyclooxygenase, which converts arachidonic acid to cyclic endoperoxides, precursors of prostaglandins. Inhibition of prostaglandin synthesis accounts for their analgesic, antipyretic, and platelet-inhibitory actions; other mechanisms may contribute to their anti-inflammatory effects. (See all compounds classified as Anti-Inflammatory Agents, Non-Steroidal.)
Cyclooxygenase Inhibitors
Compounds or agents that combine with cyclooxygenase (PROSTAGLANDIN-ENDOPEROXIDE SYNTHASES) and thereby prevent its substrate-enzyme combination with arachidonic acid and the formation of eicosanoids, prostaglandins, and thromboxanes. (See all compounds classified as Cyclooxygenase Inhibitors.)
M01AB05
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
M01AB05
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
M01AB05
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
M01AB05
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
M01AB05
S66 | EAWAGTPS | Parent-Transformation Product Pairs from Eawag | DOI:10.5281/zenodo.3754448
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AX - Other dermatologicals
D11AX18 - Diclofenac
M - Musculo-skeletal system
M01 - Antiinflammatory and antirheumatic products
M01A - Antiinflammatory and antirheumatic products, non-steroids
M01AB - Acetic acid derivatives and related substances
M01AB05 - Diclofenac
M - Musculo-skeletal system
M02 - Topical products for joint and muscular pain
M02A - Topical products for joint and muscular pain
M02AA - Antiinflammatory preparations, non-steroids for topical use
M02AA15 - Diclofenac
S - Sensory organs
S01 - Ophthalmologicals
S01B - Antiinflammatory agents
S01BC - Antiinflammatory agents, non-steroids
S01BC03 - Diclofenac
Absorption
Diclofenac is completely absorbed from the GI tract but likely undergoes significant first pass metabolism with only 60% of the drug reaching systemic circulation unchanged. Many topical formulations are absorbed percutaneous and produce clinically significant plasma concentrations. Absorption is dose proportional over the range of 25-150 mg. Tmax varies between formulations with the oral solution reaching peak plasma concentrations in 10-40min, the enteric coated tablet in 1.5-2h, and the sustained- and extended-release formulations prolonging Tmax even further. Administration with food has no significant effects on AUC but does delay Tmax to 2.5-12h.
Route of Elimination
Diclofenac is mainly eliminated via metabolism. Of the total dose, 60-70% is eliminated in the urine and 30% is eliminated in the feces. No significant enterohepatic recycling occurs.
Volume of Distribution
Diclofenac has a total volume of distribution of 5-10 L or 0.1-0.2 L/kg. The volume of the central compartment is 0.04 L/kg. Diclofenac distributes to the synovial fluid reaching peak concentration 2-4h after administration. There is limited crossing of the blood brain barrier and cerebrospinal fluid concentrations only reach 8.22% of plasma concentrations. Doses of 50 mg delivered via intramuscular injection produced no detectable diclofenac concentrations in breast milk, however metabolite concentrations were not investigated. Diclofenac has been shown to cross the placenta in mice and rats but human data is unavailable.
Clearance
Diclofenac has a plasma clearance 16 L/h.
Onset of absorption is delayed when diclofenac sodium is administered orally as delayed-release (enteric-coated) tablets, but the extent of absorption does not appear to be affected. /Diclofenac sodium/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2113
Measurable plasma concentrations of diclofenac have been observed in some fasting individuals within 10 minutes of receiving diclofenac potassium conventional tablets. /Diclofenac potassium/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2113
Diclofenac sodium and diclofenac potassium are almost completely absorbed from the GI tract; however, the drugs undergo extensive first-pass metabolism in the liver, with only about 50-60% of a dose of diclofenac sodium or diclofenac potassium reaching systemic circulation as unchanged drug. Diclofenac also is absorbed into systemic circulation following rectal administration and percutaneously following topical application to the skin as a gel or transdermal system.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2113
Food decreases the rate of absorption of conventional tablets of diclofenac potassium and of delayed-release (enteric-coated) tablets of diclofenac sodium, resulting in delayed and decreased peak plasma concentrations; however, the extent of absorption is not affected substantially. When diclofenac potassium conventional tablets are administered with food, time to achieve peak plasma concentrations of the drug is increased and peak plasma concentrations of the drug are decreased by approximately 30%. When single doses of diclofenac sodium delayed-release (enteric-coated) tablets are taken with food, the onset of absorption usually is delayed by 1-4.5 hours but may be delayed up to 12 hours in some patients. These food-induced alterations in GI absorption of the drug result from delayed transit of the delayed-release (enteric-coated) tablets to the small intestine, the site of dissolution. When diclofenac sodium extended-release tablets are taken with food, onset of absorption is delayed 1-2 hours and peak plasma concentrations are increased two-fold; however, extent of absorption is not substantially affected. Absorption of diclofenac does not appear to be affected substantially by the presence of food following continuous dosing of the drug. Antacids also may decrease the rate but not the extent of absorption of diclofenac.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2113
For more Absorption, Distribution and Excretion (Complete) data for DICLOFENAC (11 total), please visit the HSDB record page.
Diclofenac undergoes oxidative metabolism to hydroxy metabolites as well as conjugation to glucuronic acid, sulfate, and taurine. The primary metabolite is 4'-hydroxy diclofenac which is generated by CYP2C9. This metabolite is very weakly active with one thirtieth the activity of diclofenac. Other metabolites include 3'-hydroxy diclofenac, 3'-hydroxy-4'methoxy diclofenac, 4',5-dihydroxy diclofenac, an acylglucuronide conjugate, and other conjugate metabolites.
The extent of metabolism of diclofenac sodium in excised viable human skin was investigated using combination HPLC and radioactivity assay. In an earlier diffusion experiment using an in vitro flow-through diffusion system, radiolabelled diclofenac sodium in either lotion (Pennsaid) or aqueous solution was applied to viable human skin, either as single dose or multiple dose (8 times over 2 days). In this study, the receptor fluid samples from the diffusion experiment were subjected to extraction and the aliquot was analysed using HPLC to separate diclofenac and authentic metabolites. Based on the radioactivity of each HPLC fraction, the collection time of the fractions was compared with the retention time of diclofenac and metabolites in standard solutions. The samples from a single or multiple dose application of lotion showed radioactivity in mainly one fraction, whose retention time corresponded with diclofenac. Other HPLC fractions showed none or only small amounts of radioactivity within the error range of the assay. The same results were obtained with the pooled samples from the application of the lotion or of aqueous solution. The results suggest that diclofenac sodium does not undergo metabolism in viable human epidermis during percutaneous absorption in vitro. Hence, with topical application to human skin in vivo, diclofenac will be delivered with minimal, if any, metabolism. /Diclofenac sodium/
PMID:10892898 Tanojo H et al; Eur J Drug Metab Pharmacokinet 24 (4): 345-51 (1999)
In humans, metabolism of the commonly used nonsteroidal antiinflammatory drug diclofenac /compound/ 1 yields principally the 4'-hydroxy /compound/ 2, 5-hydroxy /compound/ 3, and acyl glucuronide /compound/ 4 metabolites. All three metabolites have been implicated in rare idiosyncratic adverse reactions associated with this widely used drug. Therefore, for mechanistic toxicological studies of /compound/ 1, substantial quantities of 2-4 are required and their syntheses and characterization are described here. Key steps were a convenient two-step preparation of aniline /compound/ 5 from phenol, efficient and selective 6-iodination of amide /compound/ 18, and high-yielding Ullmann couplings to generate diarylamines /compound/ 11 and /compound/ 21. The acyl glucuronide /compound/ 4 was obtained by Mitsunobu reaction of /compound/ 1 (free acid) with allyl glucuronate /compound/ 23 followed by Pd(0) deprotection, using a modification of a published procedure. /Investigators/ report full characterization of /compound/ 4 ... /Investigators/ report also the metabolic fates of the synthetic metabolites: /compound/ 2 and /compound/ 3 were glucuronidated in rats, but only /compound/ 3 formed glutathione adducts in vivo and by enzymatic synthesis via a quinoneimine intermediate. A previously undescribed glutathione adduct of /compound/ 3 was obtained by enzymatic synthesis. Compound /compound/ 4 formed an imine-linked protein conjugate as evinced by sodium cyanoborohydride trapping.
Kenny JR et al; J Med Chem 47 (11): 2816-25 (2004)
Diclofenac is eliminated predominantly (approximately 50%) as its 4'-hydroxylated metabolite in humans, whereas the acyl glucuronide (AG) pathway appears more important in rats (approximately 50%) and dogs (>80-90%). However, previous studies of diclofenac oxidative metabolism in human liver microsomes (HLMs) have yielded pronounced underprediction of human in vivo clearance. We determined the relative quantitative importance of 4'-hydroxy and AG pathways of diclofenac metabolism in rat, dog, and human liver microsomes. Microsomal intrinsic clearance values (CL(int) = V(max)/K(m)) were determined and used to extrapolate the in vivo blood clearance of diclofenac in these species. Clearance of diclofenac was accurately predicted from microsomal data only when both the AG and the 4'-hydroxy pathways were considered. However, the fact that the AG pathway in HLMs accounted for ~75% of the estimated hepatic CL(int) of diclofenac is apparently inconsistent with the 4'-hydroxy diclofenac excretion data in humans. Interestingly, upon incubation with HLMs, significant oxidative metabolism of diclofenac AG, directly to 4'-hydroxy diclofenac AG, was observed. The estimated hepatic CL(int) of this pathway suggested that a significant fraction of the intrahepatically formed diclofenac AG may be converted to its 4'-hydroxy derivative in vivo. Further experiments indicated that this novel oxidative reaction was catalyzed by CYP2C8, as opposed to CYP2C9-catalyzed 4'-hydroxylation of diclofenac. These findings may have general implications in the use of total (free + conjugated) oxidative metabolite excretion for determining primary routes of drug clearance and may question the utility of diclofenac as a probe for phenotyping human CYP2C9 activity in vivo via measurement of its pharmacokinetics and total 4'-hydroxy diclofenac urinary excretion.
PMID:12438516 Kumar S et al; J Pharmacol Exp Ther 303 (3): 969-78 (2002)
The metabolism of (14)C-diclofenac in mice was investigated following a single oral dose of 10 mg/kg. The majority of the drug-related material was excreted in the urine within 24 hr of administration (49.7%). Liquid chromatographic analysis of urine and fecal extracts revealed extensive metabolism to at least 37 components, with little unchanged diclofenac excreted. Metabolites were identified using a hybrid linear ion-trap mass spectrometer via exact mass determinations of molecular ions and subsequent multi-stage fragmentation. The major routes of metabolism identified included: 1) conjugation with taurine; and 2) hydroxylation (probably at the 4'-and 5-arene positions) followed by conjugation to taurine, glucuronic acid or glucose. Ether, rather than acyl glucuronidation, predominated. There was no evidence for p-benzoquinone-imine formation (i.e. no glutathione or mercapturic acid conjugates were detected). A myriad of novel minor drug-related metabolites were also detected, including ribose, glucose, sulfate and glucuronide ether-linked conjugates of hydroxylated diclofenac derivatives. Combinations of these hydroxylated derivatives with acyl conjugates (glucose, glucuronide and taurine) or N-linked sulfation or glucosidation were also observed. Acyl- or amide-linked-conjugates of benzoic acid metabolites and several indolinone derivatives with further hydroxylated and conjugated moieties were also evident. The mechanisms involved in the generation of benzoic acid and indolinone products indicate the formation reactive intermediates in vivo that may possibly contribute to hepatotoxicity.
PMID:21955289 Sarda S et al; Xenobiotica 42 (2): 179-94 (2012)
For more Metabolism/Metabolites (Complete) data for DICLOFENAC (7 total), please visit the HSDB record page.
Diclofenac has known human metabolites that include (2S,3S,4S,5R)-6-[2-[2-(2,6-Dichloroanilino)phenyl]acetyl]oxy-3,4,5-trihydroxyoxane-2-carboxylic acid, 4'-hydroxydiclofenac, and 5-hydroxydiclofenac.
Diclofenac is a known human metabolite of aceclofenac.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
The terminal half-life of diclofenac is approximately 2 h, however the apparent half-life including all metabolites is 25.8-33 h.
Following application of diclofenac epolamine transdermal system, the elimination half-life of diclofenac is approximately 12 hours. /Diclofenac epolamine/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2114
Following IV administration of diclofenac sodium in healthy adults, the half-life of diclofenac reportedly averages about 3 minutes in the initial distribution phase, about 16 minutes in the intermediate (redistribution) phase, and about 1-2 hours in the terminal (elimination) phase. /Diclofenac sodium/
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2114
Elimination: Up to 6 hours
Thomson.Micromedex. Drug Information for the Health Care Professional. 24th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2004., p. 377
Diclofenac inhibits cyclooxygenase-1 and -2, the enzymes responsible for production of prostaglandin (PG) G2 which is the precursor to other PGs. These molecules have broad activity in pain and inflammation and the inhibition of their production is the common mechanism linking each effect of diclofenac. PGE2 is the primary PG involved in modulation of nociception. It mediates peripheral sensitization through a variety of effects. PGE2 activates the Gq-coupled EP1 receptor leading to increased activity of the inositol trisphosphate/phospholipase C pathway. Activation of this pathway releases intracellular stores of calcium which directly reduces action potential threshold and activates protein kinase C (PKC) which contributes to several indirect mechanisms. PGE2 also activates the EP4 receptor, coupled to Gs, which activates the adenylyl cyclase/protein kinase A (AC/PKA) signaling pathway. PKA and PKC both contribute to the potentiation of transient receptor potential cation channel subfamily V member 1 (TRPV1) potentiation, which increases sensitivity to heat stimuli. They also activate tetrodotoxin-resistant sodium channels and inhibit inward potassium currents. PKA further contributes to the activation of the P2X3 purine receptor and sensitization of T-type calcium channels. The activation and sensitization of depolarizing ion channels and inhibition of inward potassium currents serve to reduce the intensity of stimulus necessary to generate action potentials in nociceptive sensory afferents. PGE2 act via EP3 to increase sensitivity to bradykinin and via EP2 to further increase heat sensitivity. Central sensitization occurs in the dorsal horn of the spinal cord and is mediated by the EP2 receptor which couples to Gs. Pre-synaptically, this receptor increases the release of pro-nociceptive neurotransmitters glutamate, CGRP, and substance P. Post-synaptically it increases the activity of AMPA and NMDA receptors and produces inhibition of inhibitory glycinergic neurons. Together these lead to a reduced threshold of activating, allowing low intensity stimuli to generate pain signals. PGI2 is known to play a role via its Gs-coupled IP receptor although the magnitude of its contribution varies. It has been proposed to be of greater importance in painful inflammatory conditions such as arthritis. By limiting sensitization, both peripheral and central, via these pathways NSAIDs can effectively reduce inflammatory pain. PGI2 and PGE2 contribute to acute inflammation via their IP and EP2 receptors. Similarly to adrenergic receptors these are Gs-coupled and mediate vasodilation through the AC/PKA pathway. PGE2 also contributes by increasing leukocyte adhesion to the endothelium and attracts the cells to the site of injury. PGD2 plays a role in the activation of endothelial cell release of cytokines through its DP1 receptor. PGI2 and PGE2 modulate T-helper cell activation and differentiation through IP, EP2, and EP4 receptors which is believed to be an important activity in the pathology of arthritic conditions. By limiting the production of these PGs at the site of injury, NSAIDs can reduce inflammation. PGE2 can cross the blood-brain barrier and act on excitatory Gq EP3 receptors on thermoregulatory neurons in the hypothalamus. This activation triggers an increase in heat-generation and a reduction in heat-loss to produce a fever. NSAIDs prevent the generation of PGE2 thereby reducing the activity of these neurons.
Diclofenac has pharmacologic actions similar to those of other prototypical NSAIAs. The drug exhibits anti-inflammatory, analgesic, and antipyretic activity. The exact mechanisms have not been clearly established, but many of the actions appear to be associated principally with the inhibition of prostaglandin synthesis. Diclofenac inhibits the synthesis of prostaglandins in body tissues by inhibiting cyclooxygenase; at least 2 isoenzymes, cyclooxygenase-1 (COX-1) and -2 (COX-2) (also referred to as prostaglandin G/H synthase-1 (PGHS-10 and -2 (PGHS-2), respectively), have been identified that catalyze the formation of prostaglandins in the arachidonic acid pathway. Diclofenac, like other prototypical NSAIAs, inhibits both COS-1 and COS-2. Although the exact mechanisms have not been clearly established, NSAIAs appear to exert anti-inflammatory, analgesic, and antipyretic activity principally through inhibition of the COS-2 isoenzyme; COX-1 inhibition presumably is responsible for the drugs' unwanted effects on GI mucosa and platelet aggregation.
American Society of Health-System Pharmacists 2011; Drug Information 2011. Bethesda, MD. 2011, p. 2112
As for all non-steroidal anti-inflammatory drugs the pharmacodynamic effects of diclofenac sodium are of anti-inflammatory, analgesic and antipyretic character due to the decrease of the prostaglandin synthesis from arachidonic acid by inhibition of the cyclo-oxygenase activity. It also induces deleterious effects on gastric and intestinal mucosa and an inhibition of platelet aggregation. /Diclofenac sodium/
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines and Inspections, Committee for Veterinary Medicinal Products; Diclofenac, Summary Report, p.1 (2003). Available from, as of October 24, 2011: https://www.ema.europa.eu/ema/index.jsp?curl=/pages/home/Home_Page.jsp&jsenabled=true
 Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
Tenatra connects Indian manufacturers with global buyers through active partners in Germany, Switzerland, Belgium, Spain & Turkey.
 Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
Delivering Quality APIs, Drug Intermediates, and Specialty Chemicals to Over 50 Countries Across the Globe.
 Cosma S.p.A., part of CFM Group with AMSA & Clarochem, provides global pharma & veterinary health with 300+ tons of FDA-approved APIs.
Cosma S.p.A., part of CFM Group with AMSA & Clarochem, provides global pharma & veterinary health with 300+ tons of FDA-approved APIs.

GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 27322
Submission : 2014-03-04
Status : Active
Type : II


GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 26224
Submission : 2012-12-17
Status : Active
Type : II


GDUFA
DMF Review : Reviewed
Rev. Date : 2014-09-11
Pay. Date : 2014-09-04
DMF Number : 15366
Submission : 2001-04-10
Status : Active
Type : II

Date of Issue : 2025-08-26
Valid Till : 2028-08-25
Written Confirmation Number : WC-0076
Address of the Firm :

NDC Package Code : 48589-0002
Start Marketing Date : 2009-11-04
End Marketing Date : 2027-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT

Registrant Name : Toru Co., Ltd.
Registration Date : 2019-12-31
Registration Number : 20190926-80-D-153-22(2)
Manufacturer Name : Unique chemicals (A Division of JB Chemicals & Pharmaceuticals Limited)
Manufacturer Address : Plot No. 5, Phase IV, GIDC Industrial Area, City : Panoli - 394 116, Dist-Bharuch, Gujarat State, India



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 19020
Submission : 2006-01-04
Status : Active
Type : II

Date of Issue : 2025-08-26
Valid Till : 2028-08-25
Written Confirmation Number : WC-0076
Address of the Firm :

Registrant Name : Kolon Pharmaceutical Co., Ltd.
Registration Date : 2019-10-30
Registration Number : 20190926-80-D-153-22(1)
Manufacturer Name : Unique chemicals (A Division of JB Chemicals & Pharmaceuticals Limited)
Manufacturer Address : Plot No. 5, Phase IV, GIDC Industrial Area, City : Panoli - 394 116, Dist-Bharuch, Gujarat State, India



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 23680
Submission : 2010-03-21
Status : Active
Type : II

Date of Issue : 2025-08-26
Valid Till : 2028-08-25
Written Confirmation Number : WC-0134
Address of the Firm :

NDC Package Code : 62512-0051
Start Marketing Date : 2015-10-18
End Marketing Date : 2026-12-31
Dosage Form (Strength) : POWDER (1kg/kg)
Marketing Category : BULK INGREDIENT



GDUFA
DMF Review : Reviewed
Rev. Date : 2014-09-12
Pay. Date : 2014-05-19
DMF Number : 28208
Submission : 2014-04-18
Status : Active
Type : II

Date of Issue : 2025-08-26
Valid Till : 2028-08-25
Written Confirmation Number : WC-0134
Address of the Firm :



GDUFA
DMF Review : N/A
Rev. Date :
Pay. Date :
DMF Number : 25107
Submission : 2011-06-30
Status : Active
Type : II


 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
REF. STANDARDS & IMPURITIES

ANALYTICAL

ABOUT THIS PAGE
71
PharmaCompass offers a list of Diclofenac API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Diclofenac manufacturer or Diclofenac supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Diclofenac manufacturer or Diclofenac supplier.
PharmaCompass also assists you with knowing the Diclofenac API Price utilized in the formulation of products. Diclofenac API Price is not always fixed or binding as the Diclofenac Price is obtained through a variety of data sources. The Diclofenac Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Voltaren manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Voltaren, including repackagers and relabelers. The FDA regulates Voltaren manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Voltaren API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Voltaren manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Voltaren supplier is an individual or a company that provides Voltaren active pharmaceutical ingredient (API) or Voltaren finished formulations upon request. The Voltaren suppliers may include Voltaren API manufacturers, exporters, distributors and traders.
click here to find a list of Voltaren suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Voltaren DMF (Drug Master File) is a document detailing the whole manufacturing process of Voltaren active pharmaceutical ingredient (API) in detail. Different forms of Voltaren DMFs exist exist since differing nations have different regulations, such as Voltaren USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Voltaren DMF submitted to regulatory agencies in the US is known as a USDMF. Voltaren USDMF includes data on Voltaren's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Voltaren USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Voltaren suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a Voltaren Drug Master File in Korea (Voltaren KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of Voltaren. The MFDS reviews the Voltaren KDMF as part of the drug registration process and uses the information provided in the Voltaren KDMF to evaluate the safety and efficacy of the drug.
After submitting a Voltaren KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their Voltaren API can apply through the Korea Drug Master File (KDMF).
click here to find a list of Voltaren suppliers with KDMF on PharmaCompass.
A Voltaren written confirmation (Voltaren WC) is an official document issued by a regulatory agency to a Voltaren manufacturer, verifying that the manufacturing facility of a Voltaren active pharmaceutical ingredient (API) adheres to the Good Manufacturing Practices (GMP) regulations of the importing country. When exporting Voltaren APIs or Voltaren finished pharmaceutical products to another nation, regulatory agencies frequently require a Voltaren WC (written confirmation) as part of the regulatory process.
click here to find a list of Voltaren suppliers with Written Confirmation (WC) on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Voltaren as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Voltaren API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Voltaren as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Voltaren and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Voltaren NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Voltaren suppliers with NDC on PharmaCompass.
Voltaren Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Voltaren GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Voltaren GMP manufacturer or Voltaren GMP API supplier for your needs.
A Voltaren CoA (Certificate of Analysis) is a formal document that attests to Voltaren's compliance with Voltaren specifications and serves as a tool for batch-level quality control.
Voltaren CoA mostly includes findings from lab analyses of a specific batch. For each Voltaren CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Voltaren may be tested according to a variety of international standards, such as European Pharmacopoeia (Voltaren EP), Voltaren JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Voltaren USP).

