



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
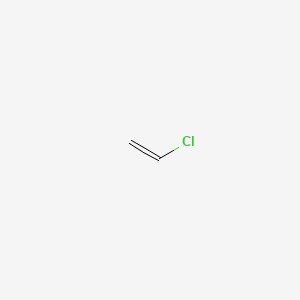

1. Chloride, Vinyl
2. Chloroethylene
1. Chloroethene
2. Chloroethylene
3. Ethene, Chloro-
4. 75-01-4
5. Monochloroethylene
6. Monochloroethene
7. Ethylene Monochloride
8. Polyvinyl Chloride
9. Chlorethylene
10. Vinylchlorid
11. Monovinyl Chloride
12. Chlorure De Vinyle
13. Ethylene, Chloro-
14. Chlorethene
15. Trovidur
16. Poly(vinyl Chloride)
17. 9002-86-2
18. Vinyl C Monomer
19. Vinyl Chloride Monomer
20. Winylu Chlorek
21. Cloruro Di Vinile
22. Rcra Waste Number U043
23. Vinyle(chlorure De)
24. Vc
25. Cloroetileno
26. Cloruro De Vinilo
27. Poly(vinylchloride)
28. Un 1086
29. Vinylchloride
30. Vcm
31. Wd06x94m2d
32. Chebi:28509
33. Vinyl Chlorine
34. Vinyl Chloride 5000 Microg/ml In Methanol
35. F-1140
36. Vinylchlorid [german]
37. Polyvinyl Chloride Resin
38. Ultron
39. Winylu Chlorek [polish]
40. Chlorure De Vinyle [french]
41. Cloruro Di Vinile [italian]
42. Ccris 621
43. Vinyle(chlorure De) [french]
44. Hsdb 169
45. Vinile (cloruro Di)
46. Vinile (cloruro Di) [italian]
47. Polyvinylchloride Latex
48. C2h3cl
49. Einecs 200-831-0
50. Un1086
51. Rcra Waste No. U043
52. Brn 1731576
53. Unii-wd06x94m2d
54. Vinyl Chloride Chloroethylene
55. Chloro-ethene
56. 1-chloroethylene
57. 1-chloroethylene #
58. Ec 200-831-0
59. Vinyl Chloride [mi]
60. 4-01-00-00700 (beilstein Handbook Reference)
61. Vinyl Chloride [hsdb]
62. Vinyl Chloride [iarc]
63. Vinyl Chloride [inci]
64. Vinyl Chloride, >=99.5%
65. Vinyl Chloride [mart.]
66. Vinyl Chloride, >=99.95%
67. Chembl2311071
68. Dtxsid8021434
69. Vinyl Chloride, Inhibited Or Vinyl Chloride Stabilized [un1086]
70. Mfcd00040415
71. Akos015916049
72. Vinyl Chloride 100 Microg/ml In Methanol
73. Vinyl Chloride 1000 Microg/ml In Methanol
74. Ft-0606106
75. Ft-0693147
76. C06793
77. C19508
78. Q338869
79. Vinyl Chloride, Inhibited Or Vinyl Chloride Stabilized
80. Vcl
| Molecular Weight | 62.50 g/mol |
|---|---|
| Molecular Formula | C2H3Cl |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 61.9923278 g/mol |
| Monoisotopic Mass | 61.9923278 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 3 |
| Formal Charge | 0 |
| Complexity | 10.3 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Carcinogens
Substances that increase the risk of NEOPLASMS in humans or animals. Both genotoxic chemicals, which affect DNA directly, and nongenotoxic chemicals, which induce neoplasms by other mechanism, are included. (See all compounds classified as Carcinogens.)
Vinyl chloride dissolved in either oil or water when administered to rats by gavage, was absorbed extremely rapidly. Peak blood serum concentrations of vinyl chloride were observed within 10 minutes of dosing.
WHO/FAO; Joint Expert Committee on Food Additives (JECFA): Food Additive Series 19: Vinyl chloride (75-01-4) (1984). Available from, as of March 26, 2018: https://www.inchem.org/documents/jecfa/jecmono/v19je16.htm
Pulmonary absorption of vinyl chloride in humans appeared to be rapid and the percentage absorbed was independent of the concentration inhaled. ... Adult male volunteers exposed for 6 hr to 2.9, 5.8, 11.6 or 23.1 ppm (7.5, 15, 30 or 60 mg/cu m) by gas mask retained on average approximately 42% of inhaled vinyl chloride. Pulmonary uptake is determined in part by the blood:air partition coefficient, which is 1.16 for vinyl chloride.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V97 389 (2008)
Animal data have demonstrated that pulmonary and gastrointestinal absorption of vinyl chloride occurs readily and rapidly. On the contrary, dermal absorption of airborne vinyl chloride is probably not significant. In monkeys, ... only 0.023-0.031% of the total available vinyl chloride was absorbed by the dermal route, whereas absorption in rats was virtually complete following single oral doses (44-92 mg/kg bw) of vinyl chloride in aqueous solution. When rats were exposed to initial concentrations of < 260 mg/cu m (100 ppm), about 40% of inhaled (14)C-vinyl chloride was absorbed by the lung.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V97 391 (2008)
The main routes of elimination of vinyl chloride and its metabolites are exhalation and urinary excretion, respectively. Accordingly, thiodiglycolic acid has been reported to be the major metabolite of vinyl chloride detected in the urine of exposed workers. Urinary levels of thiodiglycolic acid were correlated with levels of vinyl chloride in the air at concentrations of > 5 ppm.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V97 391 (2008)
For more Absorption, Distribution and Excretion (Complete) data for Vinyl chloride (18 total), please visit the HSDB record page.
INGESTED AND RECTALLY ABSORBED PVC PARTICLES (5-100 UM) WERE FOUND TO BE TRANSPORTED BY BOTH THE LYMPHATIC AND THE PORTAL SYSTEM FROM THE INTESTINAL WALL OF RATS, GUINEA-PIGS, RABBITS, CHICKENS, DOGS AND PIGS.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V19 410
PARTICLES OF POLYVINYL CHLORIDE HAVE BEEN DETECTED IN SEDIMENTS OF BLOOD, BILE, URINE, AND CEREBROSPINAL FLUID FROM ANIMALS THAT HAD BEEN FED PARTICLES OF 5-110 UM.
Clayton, G. D. and F. E. Clayton (eds.). Patty's Industrial Hygiene and Toxicology: Volume 2A, 2B, 2C: Toxicology. 3rd ed. New York: John Wiley Sons, 1981-1982., p. 4302
Vinyl chloride is primarily and rapidly metabolized in the liver, and this metabolism is saturable. ...The first step in the metabolism of vinyl chloride is oxidation, which is predominantly mediated by human cytochrome P450 (CYP) 2E1, to form the highly reactive chloroethylene oxide, which can spontaneously rearrange to chloroacetaldehyde. ... Conjugation of chloroethylene oxide and chloroacetaldehyde with glutathione (GSH) eventually leads to the major urinary metabolites N-acetyl-S-(2-hydroxyethyl)cysteine and thiodiglycolic acid. Chloroethylene oxide and chloroacetaldehyde can also be detoxified to glycolaldehyde by microsomal epoxide hydrolase (mEH) and to the urinary metabolite chloroacetic acid by aldehyde dehydrogenase 2 (ALDH2), respectively.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V97 389 (2008)
Following oral administration of (14)C-vinyl chloride, (14)C-carbon dioxide, (14)C-labelled urea and glutamic acid were identified as minor metabolites.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V97 393 (2008)
After inhalation of (14)C vinyl chloride by rats ... three urinary metabolites have been detected: N-acetyl-S-(2-hydroxyethyl)cysteine, thiodiglycolic acid, and an unidentified substance.
National Research Council. Drinking Water & Health Volume 1. Washington, DC: National Academy Press, 1977., p. 783
The principal (14)C urinary metabolites of orally administered (14)C-vinyl chloride, in the male rat, are N-acetyl-S-(2 hydroxyethyl)cysteine, N-acetyl-S-vinylcysteine and thiodiglycollic acid and lesser amounts of urea, glutamic acid, chloracetic acid and traces of methione and serine. The proportions of the three major urinary metabolites in the rat appear to be unaffected by either the dose, or the route of administration.
WHO/FAO; Joint Expert Committee on Food Additives (JECFA): Food Additive Series 19: Vinyl chloride (75-01-4) (1984). Available from, as of March 26, 2018: https://www.inchem.org/documents/jecfa/jecmono/v19je16.htm
For more Metabolism/Metabolites (Complete) data for Vinyl chloride (10 total), please visit the HSDB record page.
VINYL CHLORIDE has known human metabolites that include 2-Chlorooxirane.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Based on limited data: fairly rapid; [TDR, p. 1224]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 1224
The pattern of pulmonary elimination of 10 and 1000 ppm vinyl chloride was described by apparently similar first-order kinetics, with half-lives of 20.4 and 22.4 minutes respectively. The half lives for the initial phase of excretion of (14)C radioactivity in urine were 4.6 and 4.1 hours, respectively.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V19 392 (1979)
Vinyl chloride carcinogenicity occurs via a genotoxic pathway and is understood in some detail. Vinyl chloride is metabolized to a reactive metabolite, probably chloroethylene oxide, which is believed to be the ultimate carcinogenic metabolite of vinyl chloride. The reactive metabolite then binds to DNA, forming DNA adducts that, if not repaired, ultimately lead to mutations and tumor formation.
U.S. Environmental Protection Agency's Integrated Risk Information System (IRIS). Summary on Vinyl Chloride (75-01-4). Available from, as of March 26, 2018: https://www.epa.gov/iris/
There is a large body of data showing that VC acts as a genotoxic carcinogen. After metabolic activation to CEO /chloroethylene oxide/ by CYP2E1, VC exerts various genotoxic effects (including gene mutations and chromosomal aberrations) in different organisms, including bacteria, yeasts, mammalian cells in culture, Drosophila, rodents and humans. Among the mutagenic events induced by VC, base-pair substitutions appear, so far, to be the most frequent. VC in the presence of an activation system has a transforming activity on mammalian (rodent) cells in culture. Studies in vitro have demonstrated that metabolically activated VC and its electrophilic metabolites CEO and CAA /chloroacetaldehyde/ can alkylate nucleic acid bases. 7-OEG, the major DNA adduct formed by VC and CEO does not exhibit promutagenic properties. In contrast, four minor adducts, Epsilon A, Epsilon C, N2,3-Epsilon G and 1,N2-Epsilon G, show promutagenic properties, inducing mainly base-pair substitution mutations and a low level of frameshift mutations.
WHO/FAO; Environmental Health Criteria Document No. 215: Vinyl chloride (75-01-4) (1999). Available from, as of March 26, 2018: https://www.inchem.org/documents/ehc/ehc/ehc215.htm


