



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0

1. Cellcristin
2. Citomid
3. Farmistin
4. Leurocristine
5. Oncovin
6. Oncovine
7. Onkocristin
8. Pfs, Vincasar
9. Sulfate, Vincristine
10. Vincasar
11. Vincasar Pfs
12. Vincristin Bristol
13. Vincristin Medac
14. Vincristine
15. Vincrisul
16. Vintec
1. 2068-78-2
2. Kyocristine
3. Vincristine Sulphate
4. Vincrisul
5. Leurocristine Sulfate
6. Oncovin
7. Onkovin
8. Marqibo
9. 22-oxovincaleukoblastine Sulfate
10. Vcr Sulfate
11. Rel-vincristine Sulfate
12. Nsc 67574
13. Lilly 37231
14. Nsc67574
15. Nsc-67574
16. Leurocristine, Sulfate (1:1) (salt)
17. Alkaloid Extracted From Vinca Rosea Linn
18. Novopharm
19. Chebi:79401
20. 1217704-93-2
21. Mls002702994
22. Lilly-37231
23. Vincristini Sulfas
24. Oncovin (lilly)
25. Vincasar (tn)
26. Marqibo (tn)
27. Oncovin (tn)
28. Vincrex (tn)
29. Vincristine, Sulfate
30. Leurocristine, Sulfate
31. Vincristine Sulphate Salt
32. Vincristine Sulfate Liposome
33. Schembl3710
34. Leurocristine Sulfate (1:1)
35. Chembl501867
36. Dtxsid8044331
37. Vincristine Sulfate (jp17/usp)
38. Hms3414l13
39. Hms3678l11
40. Mfcd08706469
41. Akos015895862
42. V0129
43. Lcr
44. Vcr
45. D02197
46. 068v782
47. A936684
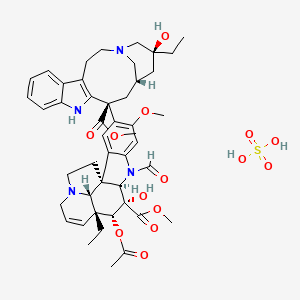
48. Methyl (3ar,3a1r,4r,5s,5ar,10br)-4-acetoxy-3a-ethyl-9-((3s,5s,7s,9s)-5-ethyl-5-hydroxy-9-(methoxycarbonyl)-1,4,5,6,7,8,9,10-octahydro-2h-3,7-methano[1]azacycloundecino[5,4-b]indol-9-yl)-6-formyl-5-hydroxy-8-methoxy-3a,3a1,4,5,5a,6,11,12-octahydro-1h-indolizino[8,1-cd]carbazole-5-carboxylate Sulfate
| Molecular Weight | 923.0 g/mol |
|---|---|
| Molecular Formula | C46H58N4O14S |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 16 |
| Rotatable Bond Count | 10 |
| Exact Mass | 922.36702371 g/mol |
| Monoisotopic Mass | 922.36702371 g/mol |
| Topological Polar Surface Area | 254 Ų |
| Heavy Atom Count | 65 |
| Formal Charge | 0 |
| Complexity | 1830 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 9 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Marqibo kit |
| Active Ingredient | Vincristine sulfate |
| Dosage Form | Injectable, liposomal |
| Route | Intravenous |
| Strength | 5mg/5ml (1mg/ml) |
| Market Status | Prescription |
| Company | Talon Therap |
| 2 of 4 | |
|---|---|
| Drug Name | Vincristine sulfate pfs |
| Active Ingredient | Vincristine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml |
| Market Status | Prescription |
| Company | Hospira; Teva Pharms Usa |
| 3 of 4 | |
|---|---|
| Drug Name | Marqibo kit |
| Active Ingredient | Vincristine sulfate |
| Dosage Form | Injectable, liposomal |
| Route | Intravenous |
| Strength | 5mg/5ml (1mg/ml) |
| Market Status | Prescription |
| Company | Talon Therap |
| 4 of 4 | |
|---|---|
| Drug Name | Vincristine sulfate pfs |
| Active Ingredient | Vincristine sulfate |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 1mg/ml |
| Market Status | Prescription |
| Company | Hospira; Teva Pharms Usa |
Antineoplastic Agents, Phytogenic
Agents obtained from higher plants that have demonstrable cytostatic or antineoplastic activity. (See all compounds classified as Antineoplastic Agents, Phytogenic.)
Tubulin Modulators
Agents that interact with TUBULIN to inhibit or promote polymerization of MICROTUBULES. (See all compounds classified as Tubulin Modulators.)


