
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
Regulatory FDF Prices
NA
0
FDF
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers

1. Bay 1021189
2. Verquvo
1. 1350653-20-1
2. Verquvo
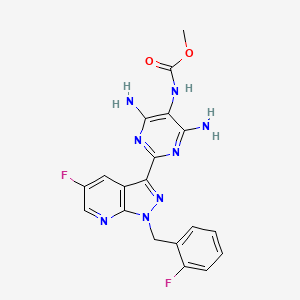
3. Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-yl)carbamate
4. Mk-1242
5. Vericiguat [inn]
6. Bay-1021189
7. Bay 1021189
8. Vericiguat [usan]
9. Bay1021189
10. Lv66adm269
11. Methyl N-[4,6-diamino-2-[5-fluoro-1-[(2-fluorophenyl)methyl]pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl]carbamate
12. Methyl (4,6-diamino-2-(5-fluoro-1-((2-fluorophenyl)methyl)-1h-pyrazolo(3,4-b)pyridin-3-yl(pyrimidin-5-yl)carbamate
13. Methyl (4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo(3,4-b)pyridin-3-yl)pyrimidin-5-yl)carbamate
14. Methyl N-(4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo(3,4-b)pyridin-3-yl)pyrimidin-5-yl)carbamate
15. Unii-lv66adm269
16. Methyl {4,6-diamino-2-[5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}carbamate
17. Vericiguatum
18. Vericiguat [jan]
19. Vericiguat [usan:inn]
20. Vericiguat [who-dd]
21. Bay1021189; Verquvo
22. Schembl429958
23. Vericiguat (jan/usan/inn)
24. Chembl4066936
25. Vericiguat [orange Book]
26. Gtpl10010
27. Chebi:142432
28. Dtxsid001318361
29. Bcp18886
30. Ex-a4694
31. Who 9805
32. Mfcd28502029
33. S9693
34. Zinc72318626
35. Cs-6981
36. Db15456
37. Sb16806
38. Ac-36737
39. Hy-16774
40. Bay1021189bay1021189
41. J3.590.750e
42. D11051
43. P14957
44. A887763
45. Q27283201
46. Bay1021189; Bay 1021189; Bay-1021189; Bay10-21189; Bay-10-21189; Bay 10-21189
47. Methyl 4,6-diamino-2-(5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl)pyrimidin-5-ylcarbamate
48. Methyl{4,6-diamino-2-[5-fluoro-1-(2-fluorobenzyl)-1h-pyrazolo[3,4-b]pyridin-3-yl]pyrimidin-5-yl}carbamate
| Molecular Weight | 426.4 g/mol |
|---|---|
| Molecular Formula | C19H16F2N8O2 |
| XLogP3 | 1.5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 5 |
| Exact Mass | 426.13642811 g/mol |
| Monoisotopic Mass | 426.13642811 g/mol |
| Topological Polar Surface Area | 147 Ų |
| Heavy Atom Count | 31 |
| Formal Charge | 0 |
| Complexity | 622 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Vericiguat is indicated in adults with symptomatic, chronic heart failure and an ejection fraction of <45% to reduce the risk of cardiovascular death and heart failure-related hospitalization following a hospitalization for heart failure or need for outpatient intravenous diuretics.
Treatment of symptomatic chronic heart failure
By directly stimulating the increased production of intracellular cyclic guanosine monophosphate (cGMP), vericiguat causes the relaxation of vascular smooth muscle and vasodilation. Vericiguat has a relatively long half-life (~30h) that allows for once-daily dosing. Animal reproduction studies have demonstrated the potential for embryo-fetal toxicity when vericiguat is administered to pregnant females - defects in major vessel and heart formation, as well as spontaneous abortions/resorptions, were observed when vericiguat was administered to pregnant rabbits during organogenesis. The possibility of pregnancy should be excluded prior to beginning therapy with vericiguat, and adequate contraception should be used throughout therapy and for one month following cessation of treatment.
C01
C - Cardiovascular system
C01 - Cardiac therapy
C01D - Vasodilators used in cardiac diseases
C01DX - Other vasodilators used in cardiac diseases
C01DX22 - Vericiguat
Absorption
Following the administration of 10mg of vericiguat by mouth once daily, the average steady-state Cmax and AUC in patients with heart failure is 350 mcg/L and 6,680 mcgh/L, respectively, with a Tmax of 1 hour. The absolute bioavailability of orally-administered vericiguat is approximately 93% when taken with food - co-administration with meals has been shown to reduce pharmacokinetic variability, increase Tmax to roughly 4 hours, and increase Cmax and AUC by 41% and 44%, respectively.
Route of Elimination
Following the oral administration of radiolabeled vericiguat, approximately 53% of the administered radioactivity was recovered in the urine and 45% in the feces. A human mass balance study found that the portion recovered in the urine comprised approximately 40.8% N-glucuronide metabolite, 7.7% other metabolites, and 9% unchanged parent drug, while virtually the entire portion recovered in the feces comprised unchanged vericiguat.
Volume of Distribution
In healthy subjects the steady-state volume of distribution of vericiguat is approximately 44 liters.
Clearance
Vericiguat is a low-clearance drug, with an observed plasma clearance of 1.6 L/h in healthy volunteers and 1.3 L/h in patients with systolic heart failure.
Vericiguat is primarily metabolized via phase II conjugation reactions, with CYP-mediated oxidative metabolism comprising a small (<5%) portion of its overall biotransformation. The major inactive metabolite, vericiguat N-glucuronide (M1), is formed by UGT1A9 and, to a lesser extent, UGT1A1. Other identified metabolites include a denbenzylated compound and an M15 metabolite thought to be the result of oxidative metabolism, although these metabolites are poorly characterized.
In patients with heart failure, the half-life of vericiguat is 30 hours.
Heart failure (HF) involves, amongst other morphologic and physiologic changes, the impaired synthesis of nitric oxide (NO) and decreased activity of soluble guanylate cyclase (sGC). Functioning normally, NO binds to sGC and stimulates the synthesis of intracellular cyclic guanosine monophosphate (cGMP), a second messenger involved in the maintenance of vascular tone, as well as cardiac contractility and remodeling. Defects in this pathway are thought to contribute to the myocardial and vascular dysfunction associated with heart failure and are therefore a desirable target in its treatment. Vericiguat directly stimulates sGC by binding to a target site on its beta-subunit, bypassing the need for NO-mediated activation, and in doing so causes an increase in the production of intracellular cGMP that results in vascular smooth muscle relaxation and vasodilation.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Global Sales Information

Market Place

Patents & EXCLUSIVITIES
ABOUT THIS PAGE
11
PharmaCompass offers a list of Vericiguat API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Vericiguat manufacturer or Vericiguat supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Vericiguat manufacturer or Vericiguat supplier.
PharmaCompass also assists you with knowing the Vericiguat API Price utilized in the formulation of products. Vericiguat API Price is not always fixed or binding as the Vericiguat Price is obtained through a variety of data sources. The Vericiguat Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Vericiguat manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Vericiguat, including repackagers and relabelers. The FDA regulates Vericiguat manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Vericiguat API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Vericiguat manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Vericiguat supplier is an individual or a company that provides Vericiguat active pharmaceutical ingredient (API) or Vericiguat finished formulations upon request. The Vericiguat suppliers may include Vericiguat API manufacturers, exporters, distributors and traders.
click here to find a list of Vericiguat suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Vericiguat DMF (Drug Master File) is a document detailing the whole manufacturing process of Vericiguat active pharmaceutical ingredient (API) in detail. Different forms of Vericiguat DMFs exist exist since differing nations have different regulations, such as Vericiguat USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Vericiguat DMF submitted to regulatory agencies in the US is known as a USDMF. Vericiguat USDMF includes data on Vericiguat's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Vericiguat USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Vericiguat suppliers with USDMF on PharmaCompass.
National Drug Code is a comprehensive database maintained by the FDA that contains information on all drugs marketed in the US. This directory includes information about finished drug products, unfinished drug products, and compounded drug products, including those containing Vericiguat as an active pharmaceutical ingredient (API).
The FDA updates the NDC directory daily. The NDC numbers for Vericiguat API and other APIs are published in this directory by the FDA.
The NDC unfinished drugs database includes product listing information submitted for all unfinished drugs, such as active pharmaceutical ingredients (APIs), drugs intended for further processing and bulk drug substances for compounding.
Pharmaceutical companies that manufacture Vericiguat as an active pharmaceutical ingredient (API) must furnish the FDA with an updated record of all drugs that they produce, prepare, propagate, compound, or process for commercial distribution in the US at their facilities.
The NDC directory also contains data on finished compounded human drug products that contain Vericiguat and are produced by outsourcing facilities. While these outsourcing facilities are not mandated to assign a Vericiguat NDC to their finished compounded human drug products, they may choose to do so.
click here to find a list of Vericiguat suppliers with NDC on PharmaCompass.
Vericiguat Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Vericiguat GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Vericiguat GMP manufacturer or Vericiguat GMP API supplier for your needs.
A Vericiguat CoA (Certificate of Analysis) is a formal document that attests to Vericiguat's compliance with Vericiguat specifications and serves as a tool for batch-level quality control.
Vericiguat CoA mostly includes findings from lab analyses of a specific batch. For each Vericiguat CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Vericiguat may be tested according to a variety of international standards, such as European Pharmacopoeia (Vericiguat EP), Vericiguat JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Vericiguat USP).

