



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
JP
0
Other Listed Suppliers
0
0
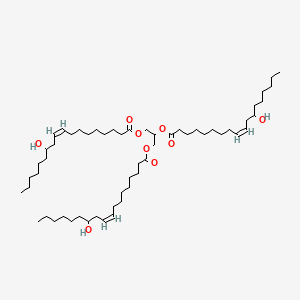

1. Oil, Castor
1. 8001-79-4
2. Ricinus Oil
3. Venelex
4. Xenaderm
5. Vegetable Oil
6. Cosmetol
7. Neoloid
8. Phorbyol
9. Ricinol
10. Ricini Oleum
11. Ricirus Oil
12. Tangantangan Oil
13. Gold Bond
14. Oleum Ricini
15. Olio Di Ricino
16. Viscotrol C
17. Crystal O
18. Palma Christi Oil
19. Aromatic Castor Oil
20. Castor Oil Aromatic
21. Db Oil
22. Ricinus Communis Oil
23. Castor Oil [jan]
24. Lavco
25. Castor Oil, Aromatic
26. Oil Of Palma Christi
27. Caswell No. 165b
28. Olio Di Ricino [italian]
29. Castor Oil, Aromatic [jan]
30. Optase
31. Unii-d5340y2i9g
32. Fema No. 2263
33. Ccris 4596
34. Hsdb 1933
35. Castor Oil (ricinus Communis L.)
36. Nci-c55163
37. Einecs 232-293-8
38. Epa Pesticide Chemical Code 031608
39. Castor Oil [usp:jan]
40. Castor Oil [oil, Edible]
41. Schembl12939325
42. 2,3-bis[[(z)-12-hydroxyoctadec-9-enoyl]oxy]propyl (z)-12-hydroxyoctadec-9-enoate
43. D5340y2i9g
44. Akos037515507
45. 1-o,2-o,3-o-tris[(z)-12-hydroxy-9-octadecenoyl]glycerol
| Molecular Weight | 933.4 g/mol |
|---|---|
| Molecular Formula | C57H104O9 |
| XLogP3 | 17.8 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 53 |
| Exact Mass | 932.76803489 g/mol |
| Monoisotopic Mass | 932.76803489 g/mol |
| Topological Polar Surface Area | 140 Ų |
| Heavy Atom Count | 66 |
| Formal Charge | 0 |
| Complexity | 1110 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 3 |
| Defined Bond Stereocenter Count | 3 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Cathartics; Emollients; Pharmaceutic Aids
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
Therapeutically, castor oil has been administered orally for its laxative action... /Former use/
Rowe, R.C., Sheskey, P.J., Quinn, M.E.; (Eds.), Handbook of Pharmaceutical Excipients 6th edition Pharmaceutical Press, London, England 2009, p. 126
Castor oil is used in cosmetics and foods and orally, parenterally, and topically in pharmaceutical formulations. It is generally regarded as a relatively nontoxic and nonirritant material when used as an excipient.
Rowe, R.C., Sheskey, P.J., Quinn, M.E.; (Eds.), Handbook of Pharmaceutical Excipients 6th edition Pharmaceutical Press, London, England 2009, p. 127
...It is usually administered only when prompt, thorough evacuation of the bowel is desired, as in preparation for certain radiological examinations. /Former/
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 925
For more Therapeutic Uses (Complete) data for Castor oil (13 total), please visit the HSDB record page.
Castor oil has been used therapeutically as a laxative and oral administration of large quantities may cause nausea, vomiting, colic, and severe purgation. It should not be given when intestinal obstruction is present.
Rowe, R.C., Sheskey, P.J., Quinn, M.E.; (Eds.), Handbook of Pharmaceutical Excipients 6th edition Pharmaceutical Press, London, England 2009, p. 127
Castor oil is contraindicated in pregnant or menstruating women.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 56:12
... Should not be taken late in the day with the expectation of sleeping.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 924
Sensitivity to castor oil when used medicinally is extremely rare.
International Labour Office. Encyclopedia of Occupational Health and Safety. Volumes I and II. New York: McGraw-Hill Book Co., 1971., p. 269
For more Drug Warnings (Complete) data for Castor oil (12 total), please visit the HSDB record page.
2(?). 2= slightly toxic: probable oral lethal dose (human): 5-15 g/kg, between 1 pint and 1 quart for 70 kg person (150 lb).
Gosselin, R.E., H.C. Hodge, R.P. Smith, and M.N. Gleason. Clinical Toxicology of Commercial Products. 4th ed. Baltimore: Williams and Wilkins, 1976., p. II-152
Indicated for over-the-counter use as a laxative for oral use and as a soothing agent for topical use on skin and hair.
Castor oil is a potent laxative that was shown to be effective for short-term constipation. In a prospective study, the group receiving oral castor oil was associated with a higher likelihood of initiation of labour compared to the placebo group. Castor oil is known to induce diarrhea, and has been used in studies to assess anti-diarrheal effect of some compounds.
Cathartics
Agents that are used to stimulate evacuation of the bowels. (See all compounds classified as Cathartics.)
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AB - Contact laxatives
A06AB05 - Castor oil
Absorption
After oral ingestion of castor oil, ricinoleic acid is released by lipases in the intestinal lumen and absorbed in the intestine. Findings from the rat study suggest that the absorption of castor oil is inversely related to the administered dose, but the absorption is virtually complete at small doses (4g).
Route of Elimination
Fecal recovery of radio-labelled castor oil ranged from 11.4% (for 10 g dose of castor oil) to 86.0% (for 44.4 g dose of castor oil).
Volume of Distribution
No pharmacokinetic data available.
Clearance
No pharmacokinetic data available.
... Some absorption of its intestinal metabolites occurs before the intestine is cleared.
Hayes, W. J., Jr. Toxicology of Pesticides Baltimore: Williams & Wilkins, 1975., p. 397
Ricinoleate, like other anionic surfactants, reduces net absorption of fluid and electrolytes and stimulates intestinal peristalsis. Ricinoleic acid also is absorbed and metabolized like other fatty acids.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 924
Two rabbits (weight = 3 kg) were fed 6% castor oil in the diet for 18 days; fecal collection occurred during the last ten days. The utilization (uncorrected for metabolic fat) of castor oil was 92.1%, which /was/ considered to be efficient utilization. For both rabbits, the percentage of fat in the feces was 2.2%.
Cosmetic Ingredient Review Expert Panel; Int J Toxicol 26 (Suppl. 3): 31-77 (2007)
Adult rats (number, weights, and strain not stated) received a diet containing 48.4% castor oil for 4 to 6 weeks. Control rats received stock ration only. Feces were collected from three rats on the castor oil diet. At the end of the feeding period, excised organs/tissues were ground thoroughly and samples of phospholipid fatty acids were obtained from the liver, small intestine, and muscle; glyceride fatty acids were obtained from the liver and fat depots. There was no evidence of catharsis in any of the animals. Average percentages of Ricinoleic Acid in the phospholipid fatty acids were as follows: liver (test: 1.3 +/- 0.6% [9 analyses]; controls: 1.7 +/- 1.1% [7 analyses]), small intestine (test: 4.9 +/- 1.7% [8 analyses]; controls: 6.0 +/- 4.4% [4 analyses]), and skeletal muscle (test: 3.6 +/- 2.9% [8 analyses]; controls: 4.0 +/- 1.7% [7 analyses]). The following values are average percentages of Ricinoleic Acid in glycerides and cholesterol esters: fat depots (test: 6.8 +/- 4.2% [11 analyses]; controls: 0.5 +/- 0.5% [7 analyses]) and liver (test: 7.2 +/- 2.4% [8 analyses]; controls: 5.6 +/- 4.1% [5 analyses]). /It was/ concluded that the feeding of castor oil did not lead to the appearance of significant amounts of Ricinoleic Acid in phospholipids of the small intestine, liver, and skeletal muscle, nor in glycerides of the liver. Additionally, they concluded that ricinoleic acid is a component acid of the glycerides in the fat depots, comprising 7% of the total fatty acids. The fatty acids excreted by each of three rats amounted to 2.1%, 2.2%, and 3.6% of those ingested. Total body fat in these three animals was also determined, and it was calculated that 1% to 2% of absorbed Ricinoleic Acid was deposited in the fat depots. /It was/ concluded that Ricinoleic Acid was rapidly metabolized.
Cosmetic Ingredient Review Expert Panel; Int J Toxicol 26 (Suppl. 3): 31-77 (2007)
For more Absorption, Distribution and Excretion (Complete) data for Castor oil (7 total), please visit the HSDB record page.
Castor oil is hydrolyzed to glycerol and ricinoleic acid via pancreatic or intestinal lipase activity. Ricinoleic acid is metabolized systemically and the metabolites are excreted. Fatty acids are expected to be degraded by pancreatic and intestinal lipase.
Castor Oil is a triglyceride that is hydrolyzed in the small intestine in humans by pancreatic enzymes, leading to the release of glycerol and Ricinoleic Acid.
Cosmetic Ingredient Review Expert Panel; Int J Toxicol 26 (Suppl. 3): 31-77 (2007)
Within the small intestine, ... pancreatic lipases hydrolyze the oil to glycerol and ricinoleic acid. Ricinoleate, like other anionic surfactants, reduces net absorption of fluid and electrolytes and stimulates intestinal peristalsis. Ricinoleic acid also is absorbed and metabolized like other fatty acids.
Hardman, J.G., L.E. Limbird, P.B. Molinoff, R.W. Ruddon, A.G. Goodman (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 9th ed. New York, NY: McGraw-Hill, 1996., p. 924
Castor oil was administered intragastrically to germ-free and conventional rats (number not stated). Urine was collected at intervals over a 24-hr period. The following epoxydicarboxylic acids were detected in the urine of both germ-free and conventional rats: 3,6-epoxyoctanedioic acid; 3,6-epoxydecanedioic acid; and 3,6- epoxydodecanedioic acid. These acids were not detected in urine collected from the rats prior to dosing with castor oil, and they also were not detected in steam-sterilized castor oil. Results for the germ-free rat indicate that the cyclization of Ricinoleic Acid (hydroxy fatty acid in castor oil) to form an epoxy compound occurs endogenously and does not require the presence of intestinal bacteria.
Cosmetic Ingredient Review Expert Panel; Int J Toxicol 26 (Suppl. 3): 31-77 (2007)
Castor oil (10 to 15 mL) /was administered/ orally to three healthy subjects. Urine was collected between 2 and 8 hr post dosing. The following three epoxydicarboxylic acids were excreted in the urine: 3,6-epoxyoctanedioic acid; 3,6-epoxydecanedioic acid; and 3,6-epoxydodecanedioic acid.
Cosmetic Ingredient Review Expert Panel; Int J Toxicol 26 (Suppl. 3): 31-77 (2007)
No pharmacokinetic data available.
Castor oil is a mix of triglycerides consisting of mainly ricinolein, linoleic acid, oleic acid, palmitic acid, stearic acid, dihydroxystearic acid, and traces of other fatty acids. The main pharmacodynamic effects of castor oil are mediated by ricinoleic acid, a hydroxylated fatty acid released from castor oil by intestinal lipases. It was believed that ricinoleic acid acts as an anionic surfactant that reduces net absorption of fluid and electrolytes, and stimulates intestinal peristalsis. However, a recent study suggests that ricinoleic acid interacts with EP3 prostanoid receptors expressed on intestinal and uterine smooth muscles. Via activating EP3 prostanoid receptors on intestinal and uterine smooth muscle cells, ricinoleic acid promotes laxation and uterus contraction, respectively. EP3 receptor act as the major prostanoid receptor in the intestine mediating propulsive effects on gut motility, and activation of EP3 receptors has been demonstrated to evoke contraction of uterine smooth muscle.
Castor oil has been classified as a stimulant because lipolysis in small intestine liberates ricinoleic acid ... /which/ stimulates smooth muscle and inhibits the absorption of water and electrolytes resulting in fluid accumulation in vitro, but it is not known whether these changes affect fluid movement or...laxative effect in vivo.
American Medical Association, AMA Department of Drugs. AMA Drug Evaluations. 4th ed. Chicago: American Medical Association, 1980., p. 982
In a study involving male Crl:CD BR rats, the findings suggested that castor oil-induced diarrhea is the result of activation of NK1 and NK2 receptors by endogenous tachykinins.
Cosmetic Ingredient Review Expert Panel; Int J Toxicol 26 (Suppl. 3): 31-77 (2007)


