


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
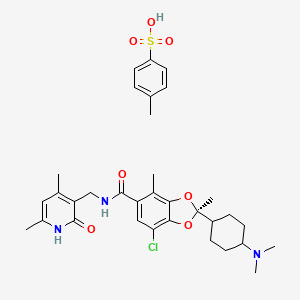

1. Valemetostat Tosylayte
2. 1809336-93-3
3. Valemetostat (tosylate)
4. 6n79i7x5ii
5. 1809336-93-3 (tosylate)
6. Valemetostat Tosilate (jan)
7. Valemetostat Tosilate [jan]
8. (2r)-7-chloro-2-[trans-4-(dimethylamino)cyclohexyl]-n-[(4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide Tosylate
9. 1,3-benzodioxole-5-carboxamide, 7-chloro-n-((1,2-dihydro-4,6-dimethyl-2-oxo-3-pyridinyl)methyl)-2-(trans-4-(dimethylamino)cyclohexyl)-2,4-dimethyl-, (2r)-, Compd. With 4-methylbenzenesulfonate (1:1)
10. Ds-3201 Tosylate
11. Valemetostat Tosilate
12. Unii-6n79i7x5ii
13. Chembl4594405
14. Schembl18393873
15. Hy-109108a
16. Cs-0101884
17. D11662
18. (2r)-7-chloro-2-[4-(dimethylamino)cyclohexyl]-n-[(4,6-dimethyl-2-oxo-1h-pyridin-3-yl)methyl]-2,4-dimethyl-1,3-benzodioxole-5-carboxamide;4-methylbenzenesulfonic Acid
19. (r)-7-chloro-n-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-((1r,4r)-4-(dimethylamino)cyclohexyl)-2,4-dimethylbenzo[d][1,3]dioxole-5-carboxamide 4-methylbenzenesulfonate
20. (r)-7-chloro-n-((4,6-dimethyl-2-oxo-1,2-dihydropyridin-3-yl)methyl)-2-((1r,4r)-4-(dimethylamino)cyclohexyl)-2,4-dimethylbenzo[d][1,3]dioxole-5-carboxamide4-methylbenzenesulfonate
| Molecular Weight | 660.2 g/mol |
|---|---|
| Molecular Formula | C33H42ClN3O7S |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 6 |
| Exact Mass | 659.2431996 g/mol |
| Monoisotopic Mass | 659.2431996 g/mol |
| Topological Polar Surface Area | 143 Ų |
| Heavy Atom Count | 45 |
| Formal Charge | 0 |
| Complexity | 1090 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |


