



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
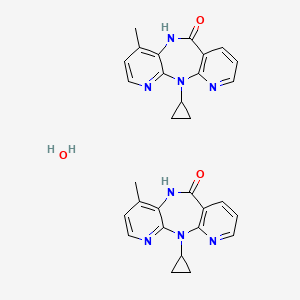

1. Bi Rg 587
2. Bi-rg-587
3. Birg587
4. Hemihydrate, Nevirapine
5. Nevirapine
6. Viramune
1. 220988-26-1
2. B7xf2td73c
3. 2-cyclopropyl-7-methyl-2,4,9,15-tetrazatricyclo[9.4.0.03,8]pentadeca-1(11),3,5,7,12,14-hexaen-10-one;hydrate
4. Unii-b7xf2td73c
5. Schembl1923903
6. Dtxsid30176606
7. Nevirapine Hemihydrate [usp-rs]
8. Nevirapine Hemihydrate [who-dd]
9. Nevirapine Hemihydrate [who-ip]
10. Nevirapine Hemihydrate [ep Monograph]
11. Nevirapine Hemihydrate [usp Monograph]
12. Nevirapinum Hemihydrate [who-ip Latin]
13. Q27274473
14. 6h-dipyrido(3,2-b:2',3'-e)(1,4)diazepin-6-one, 11-cyclopropyl-5,11-dihydro-4-methyl-, Hydrate (2:1)
| Molecular Weight | 550.6 g/mol |
|---|---|
| Molecular Formula | C30H30N8O3 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 2 |
| Exact Mass | 550.24408685 g/mol |
| Monoisotopic Mass | 550.24408685 g/mol |
| Topological Polar Surface Area | 117 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 397 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
Cytochrome P-450 CYP3A Inducers
Drugs and compounds that induce the synthesis of CYTOCHROME P-450 CYP3A. (See all compounds classified as Cytochrome P-450 CYP3A Inducers.)
Reverse Transcriptase Inhibitors
Inhibitors of reverse transcriptase (RNA-DIRECTED DNA POLYMERASE), an enzyme that synthesizes DNA on an RNA template. (See all compounds classified as Reverse Transcriptase Inhibitors.)




