



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
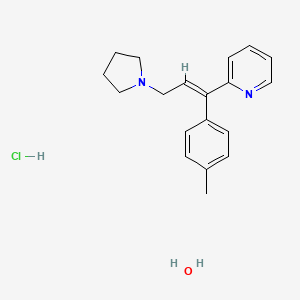

1. Actidil
2. Anhydrous, Triprolidine Hydrochloride
3. Hydrochloride Anhydrous, Triprolidine
4. Hydrochloride, Triprolidine
5. Pro Actidil
6. Triprolidine
7. Triprolidine Hydrochloride
8. Triprolidine Hydrochloride Anhydrous
9. Triprolidine Monohydrochloride
10. Triprolidine Monohydrochloride, (z)-isomer
11. Triprolidine Monohydrochloride, Monohydrate
12. Triprolidine Oxalate
13. Triprolidine Oxalate, (trans)-isomer
14. Triprolidine, (z)-isomer
1. Triprolidine Hcl
2. 6138-79-0
3. Actidil
4. Myidyl
5. Triprolidine.hcl Monohydrate
6. Trans-triprolidinehydrochloride
7. Triprolidine Hydrochloride Hydrate
8. Yan7r5l890
9. Chebi:32265
10. (e)-2-(3-(1-pyrrolidinyl)-1-p-tolylpropenyl)pyridine Monohydrochloride Monohydrate
11. Nsc-757361
12. Pro-actidil
13. Pro-entra
14. Pyridine, 2-(1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl)-, Monohydrochloride, Monohydrate, (e)-
15. 2-[(1e)-1-(4-methylphenyl)-3-pyrrolidin-1-ylprop-1-en-1-yl]pyridine Hydrochloride Hydrate
16. Triprolidine Hydrochloride Hydrate (jan)
17. Pyridine, 2-[1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl]-, Monohydrochloride, Monohydrate, (e)-
18. Ccris 7215
19. Triprolidine Hydrochloride Hydrate [jan]
20. Triprolidine (hydrochloride Monohydrate)
21. Unii-yan7r5l890
22. Actidilon
23. Triprolidine Hydrochloride [usp:jan]
24. Venen (tn)
25. Dsstox_cid_1410
26. Dsstox_rid_76146
27. Dsstox_gsid_21410
28. Triprolidinehydrochloridehydrate
29. Schembl1140974
30. Chembl3188034
31. Dtxsid0021410
32. Triprolidine Hydrochloride (usp)
33. Triprolidinhydrochlorid Monohydrat
34. Hy-b1301
35. Tox21_200848
36. S5447
37. Akos024370814
38. Ccg-267838
39. Nsc 757361
40. W38t790
41. (e)-2-(3-(pyrrolidin-1-yl)-1-(p-tolyl)prop-1-en-1-yl)pyridine Hydrochloride Hydrate
42. Ncgc00258402-01
43. Bs-17733
44. Pyridine, 2-(3-(1-pyrrolidinyl)-1-p-tolylpropenyl)-, Monohydrochloride, Monohydrate, Stereoisomer
45. Triprolidine Hydrochloride [mart.]
46. Triprolidine Hydrochloride [vandf]
47. Cas-6138-79-0
48. Triprolidine Hydrochloride [usp-rs]
49. Cs-0013069
50. D01782
51. D70442
52. Triprolidine Hydrochloride [orange Book]
53. Myfed Component Triprolidine Hydrochloride
54. Actifed Component Triprolidine Hydrochloride
55. Corphed Component Triprolidine Hydrochloride
56. Triphed Component Triprolidine Hydrochloride
57. Triprolidine Hydrochloride [usp Monograph]
58. Triprolidine Hydrochloride Monohydrate [mi]
59. Actahist Component Triprolidine Hydrochloride
60. Allerfed Component Triprolidine Hydrochloride
61. Histafed Component Triprolidine Hydrochloride
62. Trilitron Component Triprolidine Hydrochloride
63. Triprolidine Hydrochloride Component Of Myfed
64. Q27114841
65. Triacin-c Component Triprolidine Hydrochloride
66. Triprolidine Hydrochloride Component Of Actahist
67. Triprolidine Hydrochloride Component Of Actifed
68. Triprolidine Hydrochloride Component Of Allerfed
69. Triprolidine Hydrochloride Component Of Corphed
70. Triprolidine Hydrochloride Component Of Histafed
71. Triprolidine Hydrochloride Component Of Triphed
72. Triprolidine Hydrochloride Monohydrate [mart.]
73. Triprolidine Hydrochloride Monohydrate [who-dd]
74. Triprolidine Hydrochloride Component Of Triacin-c
75. Triprolidine Hydrochloride Component Of Trilitron
76. Triprolidine Hydrochloride Monohydrochloride Monohydrate
77. (e)-2-[3-(pyrrolidin-1-yl)-1-p-tolylprop-1-enyl]pyridine Hydrochloride Monohydrate
78. 1-[(2e)-3-(4-methylphenyl)-3-(pyridin-2-yl)prop-2-en-1-yl]pyrrolidinium Chloride--water (1/1)
79. 2-[(1e)-1-(4-methylphenyl)-3-(pyrrolidin-1-yl)prop-1-en-1-yl]pyridine Hydrochloride--water (1/1)
| Molecular Weight | 332.9 g/mol |
|---|---|
| Molecular Formula | C19H25ClN2O |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 4 |
| Exact Mass | 332.1655411 g/mol |
| Monoisotopic Mass | 332.1655411 g/mol |
| Topological Polar Surface Area | 17.1 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 336 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Anti-Allergic Agents
Agents that are used to treat allergic reactions. Most of these drugs act by preventing the release of inflammatory mediators or inhibiting the actions of released mediators on their target cells. (From AMA Drug Evaluations Annual, 1994, p475) (See all compounds classified as Anti-Allergic Agents.)
Histamine H1 Antagonists
Drugs that selectively bind to but do not activate histamine H1 receptors, thereby blocking the actions of endogenous histamine. Included here are the classical antihistaminics that antagonize or prevent the action of histamine mainly in immediate hypersensitivity. They act in the bronchi, capillaries, and some other smooth muscles, and are used to prevent or allay motion sickness, seasonal rhinitis, and allergic dermatitis and to induce somnolence. The effects of blocking central nervous system H1 receptors are not as well understood. (See all compounds classified as Histamine H1 Antagonists.)


