


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
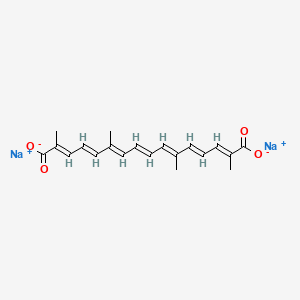

1. (2e,4e,6e,8e,10e,12e,14e)-2,6,11,15-tetramethyl-2,4,6,8,10,12,14-hexadecaheptaenedioic Acid
2. Crocetin
3. Crocetin Sodium Salt
4. Trans-sodium Crocetinate
5. Transcrocetinate Sodium
6. Tsc
7. Tsc Cpd
1. 591230-99-8
2. Transcrocetinate Sodium
3. Disodium Trans-crocetinate
4. Transcrocetinate Disodium
5. Trans-sodium Crocetinate
6. Trans-crocetin Sodium
7. Transcrocetinate Sodium [usan]
8. Yp57637wmx
9. 591230-99-8 (sodium 1
10. Disodium (all-e)-2,6,11,15-tetramethylhexadecahepta-2,4,6,8,10,12,14-enedioate
11. 64603-92-5
12. 2,4,6,8,10,12,14-hexadecaheptaenedioic Acid, 2,6,11,15-tetramethyl-, Sodium Salt (1:2), (2e,4e,6e,8e,10e,12e,14e)-
13. Sodium (2e,4e,6e,8e,10e,12e,14e)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate
14. Unii-yp57637wmx
15. Crocetin Sodium
16. Chembl3137335
17. Akos037515031
18. Transcrocetinate Sodium [who-dd]
19. E80767
20. Q27294636
21. Transcrocetinate Disodium (disodium Trans-crocetinate)
22. Disodium;(2e,4e,6e,8e,10e,12e,14e)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate
| Molecular Weight | 372.4 g/mol |
|---|---|
| Molecular Formula | C20H22Na2O4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 372.13134774 g/mol |
| Monoisotopic Mass | 372.13134774 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 597 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 7 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Anticarcinogenic Agents
Agents that reduce the frequency or rate of spontaneous or induced tumors independently of the mechanism involved. (See all compounds classified as Anticarcinogenic Agents.)
Antioxidants
Naturally occurring or synthetic substances that inhibit or retard oxidation reactions. They counteract the damaging effects of oxidation in animal tissues. (See all compounds classified as Antioxidants.)


