



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
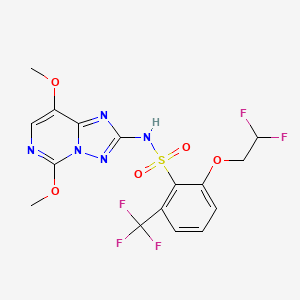

1. 219714-96-2
2. Granite
3. 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide
4. Penoxsulam [iso]
5. 784elc1scz
6. Chebi:81776
7. 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide
8. 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy(1,2,4)triazolo(1,5-c)pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide
9. Pxd
10. Viper
11. Penoxsulam [mi]
12. Unii-784elc1scz
13. Dsstox_cid_14803
14. Dsstox_rid_79204
15. Dsstox_gsid_34803
16. Schembl116968
17. Chembl1895913
18. Dtxsid0034803
19. Hsdb 7887
20. Amy12535
21. Bcp18718
22. Tox21_301010
23. De 638
24. De-638
25. Mfcd07363876
26. Zinc13827750
27. Akos025401685
28. Penoxsulam 100 Microg/ml In Methanol
29. Ncgc00163715-01
30. Ncgc00163715-02
31. Ncgc00163715-03
32. Ncgc00254912-01
33. Ac-24494
34. Penoxsulam 100 Microg/ml In Acetonitrile
35. Penoxsulam 1000 Microg/ml In Acetonitrile
36. Cas-219714-96-2
37. Ft-0696708
38. Penoxsulam, Pestanal(r), Analytical Standard
39. C13421
40. C18481
41. Q22808507
42. 2-(2,2-difluoroethoxy)-6-trifluoromethyl-n-(5, 8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)benzenesulfonamide
43. 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy[1,2,4]-triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide
44. 2-(2,2-difluoroethoxy)-n-{5,8-dimethoxy-[1,2,4]triazolo[1,5-c]pyrimidin-2-yl}-6-(trifluoromethyl)benzene-1-sulfonamide
45. 2-(2,2-difluoroethyl)-n-(5,8-dimethoxy[1,2,4]triazolo[1,5-c]pyrimidin-2-yl)-6-(trifluoromethyl)benzenesulfonamide
46. 3-(2,2-difluoroethoxy)-n-(5,8-dimethoxy(1,2,4)triazolo(1,5-c)pyrimidin-2-yl)-.alpha.,.alpha.,.alpha.-trifluorotoluene-2-sulfonamide
47. Benzenesulfonamide, 2-(2,2-difluoroethoxy)-n-(5,8-dimethoxy(1,2,4)triazolo(1,5-c)pyrimidin-2-yl)-6-(trifluoromethyl)-
| Molecular Weight | 483.4 g/mol |
|---|---|
| Molecular Formula | C16H14F5N5O5S |
| XLogP3 | 2.9 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 14 |
| Rotatable Bond Count | 8 |
| Exact Mass | 483.06358055 g/mol |
| Monoisotopic Mass | 483.06358055 g/mol |
| Topological Polar Surface Area | 125 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 727 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
All dosing was by gavage with labeled penoxsulam (XDE-638) (97.5% purity of unlabeled penoxsulam, triazole label purity > 99%, phenyl ring label purity 98.4%) in 0.5% Methocel suspension. Generally, groups of 4 rats/sex were used in each of 8 tests using triazole-labeled 14C-XDE-638. One additional set of 4 males was tested using phenyl-labeled 14C-XDE-638 to assess the extent of cleavage between triazole and phenyl moieties. Rats fitted with jugular vein cannulae were used primarily to determine the time course of residue concentrations in plasma, urine, and feces after single dosing with either 5 or 250 mg/kg penoxsulam. These rats were sacrificed on day 7 (as were the phenyl-labeled males), and tissue residues were determined at that time. Time to maximal plasma concentration (Cmax) was about 0.5 hr and 2 hr for both sexes at 5 and 250 mg/kg, respectively. Time to half of maximal concentration of radiolabel (1/2 Cmax) at 5 and 250 mg/kg were determined to be 2.6 hr and 3.0 hr for males, respectively, and 2.9 hr and 5.6 hr for females, respectively. Thus four sets of males and females were sacrificed following single oral dosing to achieve approximately Cmax and 1/2 Cmax plasma levels at the two dose levels, and to obtain tissue and excreta samples at early stages of exposure. Radiolabel was measured in each of 24 tissues at each sacrifice time. Quantities of major metabolites were assessed in pooled plasma at various intervals, and in liver and kidney tissue at sacrifice times. Three rats/sex were fitted with bile duct cannulae, and bile was collected at intervals over 24 hr to assess biliary excretion rate and metabolite analysis. Four additional rats/sex were dosed daily for 15 days with 5 mg/kg/day prior to treatment with triazole labeled 14C-XDE-638 on day 16. These rats were evaluated for residues in excreta and for tissue levels of metabolites, which were found to be comparable to non-pre-treated rats. Metabolite separation was by HPLC using C-18 stationary phase and a gradient program sufficient to separate most major peaks for detection by UV (254 nm) and by 14C-detector. Metabolite identification was by retention time and negative ion LC/MS. Estimated total absorption after 5 mg/kg was about 85% for either sex). Absorption was much lower after 250 mg/kg (17% in males, 32% in females). Unless otherwise stated, results below derive from single-dose administration of 5 mg/kg triazole-labeled penoxsulam. A comparison of distribution patterns of urinary and fecal metabolites following phenyl- or triazole-label indicated that most (at least 90%) of penoxsulam residues remained intact between the two labeled rings. Parent compound was the most abundant urinary residue, constituting about 31% and 19% of administered dose in urine samples of males dosed with triazole- or phenyl-labeled 14C, respectively. Females consistently excreted much larger percentages of label in urine than did males. In the 7-day urine collection in females, 66% of administered dose was parent penoxsulam. The most abundant fecal metabolite in either sex in the 7-day collection was an uncharacterized 'Metabolite Y,' comprising 14% to 19% of administered dose in males and 6% in females. Parent penoxsulam constituted 12% to 15% of administered label in feces of males and 3% in females. About 88% of 24-hr fecal label in males represented absorbed penoxsulam, based on percentage of label excreted in the bile. As expected, considering the proportionally higher excretion of label in females via the urine, females excreted much less in 24-hr bile collections than did males (14% of administered dose in females vs. 56% of administered dose in males). The largest single provisionally identified component of bile was the glutathione product of 5-hydroxy- or 8-hydroxy-penoxsulam (18% of administered penoxsulam). Two glucuronide products of hydroxylated penoxsulam (position of hydroxylation product unknown in either case) appeared to account together for about 15% of administered label in bile. Tissue concentration evaluations during times of 1/2 Cmax plasma concentrations revealed relatively high initial liver residue concentrations, whereas other tissues (except for 'GI/Ingesta') had much lower levels. Radiolabel in liver and in all other tissues were very low by day 7. Parent penoxsulam was much more abundant than any other residues in plasma, liver, and kidneys. Comparative concentrations of penoxsulam in ug-equivalents/g tissue at the time of plasma 1/2 Cmax for these tissues in males and females, respectively, were 10 and 9 for plasma, 44 and 50 for liver, and 3 and 4 for kidney. Originally, this study was unacceptable but upgradeable with identification of 'Metabolite Y,' which constituted up to 19% of fecal residues
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data on Penoxsulam p.11 (2005). Available from, as of February 7, 2011: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
In a metabolism study in rats, (14)C-penoxsulam was rapidly and nearly completely absorbed at the low dose of 5.0 mg/kg, but at the high dose of 250 mg/kg, there was evidence that absorption was largely incomplete (i.e. absorption was saturated). Both gender and dose affected the excretion pattern. At the low dose, the major route of excretion of radioactivity was via the feces in males and via the urine in females. At the high dose, radioactivity was predominantly excreted via the feces in both sexes. A significant enterohepatic circulation was observed, particularly in males. Most (>90%) of the administered dose was excreted within 36-48 hours. There was negligible radioactivity in tissues at 7 days and no evidence of accumulation in any tissue/organ. Although numerous metabolites were revealed in the urine, feces and bile, nearly all were <1% of the administered dose. Parent compound and a 2-hydroxyphenyl derivative were the major compounds in urine and feces.
US EPA Office of Prevention, Pesticides and Toxic Substances, Pesticide Fact Sheet for Penoxsulam, p.5 (September 2004). Available from, as of February 4, 2011: https://www.epa.gov/opprd001/factsheets/
All dosing was by gavage with labeled penoxsulam (XDE-638) (97.5% purity of unlabeled penoxsulam, triazole label purity > 99%, phenyl ring label purity 98.4%) in 0.5% Methocel suspension. Generally, groups of 4 rats/sex were used in each of 8 tests using triazole-labeled 14C-XDE-638. One additional set of 4 males was tested using phenyl-labeled 14C-XDE-638 to assess the extent of cleavage between triazole and phenyl moieties. Rats fitted with jugular vein cannulae were used primarily to determine the time course of residue concentrations in plasma, urine, and feces after single dosing with either 5 or 250 mg/kg penoxsulam. ... Quantities of major metabolites were assessed in pooled plasma at various intervals, and in liver and kidney tissue at sacrifice times. Three rats/sex were fitted with bile duct cannulae, and bile was collected at intervals over 24 hr to assess biliary excretion rate and metabolite analysis. Four additional rats/sex were dosed daily for 15 days with 5 mg/kg/day prior to treatment with triazole labeled 14C-XDE-638 on day 16. These rats were evaluated for residues in excreta and for tissue levels of metabolites, which were found to be comparable to non-pre-treated rats. Metabolite separation was by HPLC using C-18 stationary phase and a gradient program sufficient to separate most major peaks for detection by UV (254 nm) and by 14C-detector. Metabolite identification was by retention time and negative ion LC/MS. ... A comparison of distribution patterns of urinary and fecal metabolites following phenyl- or triazole-label indicated that most (at least 90%) of penoxsulam residues remained intact between the two labeled rings. Parent compound was the most abundant urinary residue, constituting about 31% and 19% of administered dose in urine samples of males dosed with triazole- or phenyl-labeled 14C, respectively. ... The most abundant fecal metabolite in either sex in the 7-day collection was an uncharacterized 'Metabolite Y,' comprising 14% to 19% of administered dose in males and 6% in females. Parent penoxsulam constituted 12% to 15% of administered label in feces of males and 3% in females. ... The largest single provisionally identified component of bile was the glutathione product of 5-hydroxy- or 8-hydroxy-penoxsulam (18% of administered penoxsulam). Two glucuronide products of hydroxylated penoxsulam (position of hydroxylation product unknown in either case) appeared to account together for about 15% of administered label in bile. ...
California Environmental Protection Agency/Department of Pesticide Regulation; Summary of Toxicology Data on Penoxsulam p.11 (2005). Available from, as of February 7, 2011: https://www.cdpr.ca.gov/docs/risk/toxsums/toxsumlist.htm
Sulfonamide herbicides ... inhibit the enzyme acetolactate synthase (ALS)... /Sulfonamides/
PMID:19464188 Johnson TC et al; Bioorg Med Chem 17 (12): 4230-40 (2009)


