



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
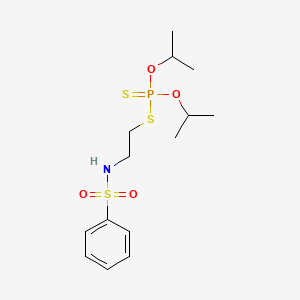

1. 741-58-2
2. Betasan
3. Benzulfide
4. Prefar
5. Disan
6. Sap (herbicide)
7. Disan (pesticide)
8. R 4461
9. N-[2-di(propan-2-yloxy)phosphinothioylsulfanylethyl]benzenesulfonamide
10. O,o-diisopropyl 2-(benzenesulfonamido)ethyl Dithiophosphate
11. Chebi:81899
12. O,o-diisopropyl S-(2-benzenesulfonylaminoethyl) Phosphorodithioate
13. 9882bw2q2s
14. N-(2-mercaptoethyl)benzenesulfonamide S-(o,o-diisopropyl Phosphorodithioate)
15. Phosphorodithioic Acid, O,o-diisopropyl Ester, S-ester With N-(2-mercaptoethyl)benzenesulfonamide
16. Sap
17. Kayaphenone
18. Betamec
19. Exporsan
20. Prefer
21. N-(2-(o,o-diisopropyldithiophosphoryl)ethyl)benzenesulfonamide
22. Betasan E
23. Betasan G
24. Prefar E
25. Pre-san
26. Phosphorodithioic Acid, O,o-bis(1-methylethyl) S-(2-((phenylsulfonyl)amino)ethyl) Ester
27. Phosphorodithioic Acid, O,o-bis(1-methylethyl) S-[2-[(phenylsulfonyl)amino]ethyl] Ester
28. Caswell No. 357
29. Betasan [obsolete]
30. Bensumec
31. Bensulide [bsi:iso]
32. Hsdb 393
33. Einecs 212-010-4
34. Epa Pesticide Chemical Code 009801
35. Brn 2164989
36. (n-(2-mercaptoethyl)benzenesulfonamide)
37. Bensulfide
38. N-[2-(o,o-diisopropyldithiophosphoryl)ethyl]benzenesulfonamide
39. Unii-9882bw2q2s
40. Ccris 9246
41. O,o-diisopropyl S-{2-[(phenylsulfonyl)amino]ethyl} Dithiophosphate
42. R-4461
43. Bensulide [iso]
44. N-(beta-o,o-diisopropyldithiophosphorylethyl)benzenesulfonamide
45. S-2-benzenesulfonamidoethyl O,o-di-isopropyl Phosphorodithioate
46. S-2-benzenesulphonamidoethyl O,o-diisopropyl Phosphorodithioate
47. S-beta-(benzenesulfonamido)ethyl O,o-diisopropyl Dithiophosphate
48. Bensulide [hsdb]
49. O,o-di-isopropyl S-2-phenylsulphonylaminoethyl Phosphorodithioate
50. N-(2-ethylthio)benzene Sulphonamide-s,o,o-diisopropylphosphorodithioate
51. N-(2-mercaptoethylbenzene)sulfonamide S-(o,o-diisopropylphosphorodithioate)
52. O,o-bis(1-methylethyl) S-(2-((phenylsulfonyl)amino)ethyl)pheosphorodithioate
53. O,o-bis(1-methylethyl)-s-(2-((phenylsulfonyl)amino)ethyl) Phosphorodithioate
54. S-(o,o-diisopropylphosphorodithioate) Of N-(2-mercaptoethyl)benzenesulfonamide
55. Benzenesulfonamide, N-(2-mercaptoethyl)-, S-ester With O,o-diisopropylphosphorodithioate
56. Dsstox_cid_12329
57. Dsstox_rid_78915
58. O,o-diisopropyl Dithiophosphate S-ester With N-(2-mercaptoethyl)benzenesulphonamide
59. O,o-diisopropyl Phosphorodithioate S-ester With N-(2-mercaptoethyl)benzenesulfonamide
60. O,o-diisopropyl Phosphorothioate S-ester With N-(2-mercaptoethyl)benzenesulfonamide
61. S-(o,o-diisopropyl Phosphorodithioate) Ester Of N-(2-mercaptoethyl)benzenesulfonamide
62. Dsstox_gsid_32329
63. Schembl55205
64. Chembl1895252
65. Dtxsid9032329
66. Tox21_300733
67. Bensulide 100 Microg/ml In Methanol
68. Akos015902619
69. O,o-bis(1-methylethyl) S-(2-((phenylsulfonyl)amino)ethyl)phosphorodithioate
70. Bensulide 1000 Microg/ml In Methanol
71. Bensulide 10 Microg/ml In Acetonitrile
72. Ncgc00168292-01
73. Ncgc00168292-02
74. Ncgc00168292-03
75. Ncgc00168292-04
76. Ncgc00254639-01
77. Cas-741-58-2
78. Bensulide, Pestanal(r), Analytical Standard
79. C18703
80. Q414584
81. O,o'-diisopropyl S-[2-(benzenesulfonamido)ethyl]dithiophosphate
82. O,o'-diisopropyl S-[2-(benzenesulfonamido)ethyl]phosphorodithioate
83. O,o-diisopropyl S-(2-benzenesulfonylaminoethyl)phosphorodithioate
84. S-.beta.-(benzenesulfonamido)ethyl O,o-diisopropyl Dithiophosphate
85. O,o-diisopropyl S-(2-[(phenylsulfonyl)amino]ethyl) Dithiophosphate #
86. (n-(2-ethylthio)benzene Sulfonamido-s,o,o- Diisopropylphosphorodithioate
87. Phosphorodithioic Acid O,o-bis(1-methylethyl) S-[2-[(phenylsulfonyl)amino]ethyl] Ester
88. Phosphorodithioic Acid-o,o-bis(1-methylethyl)-s-(2-((phenylsulfonyl)amino)ethyl)ester
| Molecular Weight | 397.5 g/mol |
|---|---|
| Molecular Formula | C14H24NO4PS3 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 10 |
| Exact Mass | 397.06050877 g/mol |
| Monoisotopic Mass | 397.06050877 g/mol |
| Topological Polar Surface Area | 130 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 471 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Selective herbicide, adsorbed on the root surfaces, and a small amount is absorbed by the roots. Translocation of bensulide to the leaves does not occur, but metabolites are translocated.
Tomlin, C.D.S. (ed.). The Pesticide Manual - World Compendium. 10th ed. Surrey, UK: The British Crop Protection Council, 1994., p. 87
In a metabolism study, bensulide technical, labelled with C in the 14 phenyl ring (> 96.4% radiopurity; 925 MBq/mMole) was dissolved in corn oil (vehicle) and administered to Sprague-Dawley rats (5/sex/group; 7-8 weeks of age; 185-235 g body weight) following 3 treatment regimes. Animals in Group I received a single oral dose of radioactive bensulide at 1 mg/kg bw. Animals in Group II received 14 consecutive doses (1 mg/kg/day) of non-radioactive bensulide technical (99% a.i.) in corn oil, followed by a 1 mg/kg dose of radiolabelled bensulide technical in corn oil on day 15. Group III animals received a single oral dose of radiolabelled bensulide technical at 100 mg/kg bw. An additional group of animals (Group IV; 3/sex/group) were given a single oral dose of radiolabelled bensulide 18 technical at 1 mg/kg bw and were subseqently used for autoradiological radiolabelled carbon dioxide release determinations. ... In Group I, total urinary excretion of 7 days after admin of radioactive bensulide technical accounted for 70 and 75% of the administered dose in males and females, respectively. ... In Group III, total urinary excretion accounted for 75 and 87% of the administered dose in males and females, respectively. ... For Group II (prior 14-day admin of non-radioactive bensulide technical before radioactive bensulide admin, both at 1 mg/kg), total urinary excretion of radioactivity over 7 days past dosing with radioactive bensulide accounted for 79 and 88% of the administered dose in males and females, respectively. For Group IV, urinary excretion of 14-C radioactivity derived from bensulide technical over a 48-hr period accounted for 67% for one male and 86% in one male. For Group I, total fecal excretion of radioactivity derived from 14C-bensulide technical over 7 days post-dosing accounted for 22 and 20% of the administered dose in males and females, respectively. ... For Group III, total fecal elim over 7 days post-dosing of bensulide-derived radioactivity accounted for 22 and 11% of the administered dose for males and females, respectively. ... In Group II animals, total fecal excretion of radioactivity over 7 days post-dosing accounted for 14 and 8% of the administered dose for males and females, respectively. ... In Group IV, fecal excretion of radioactivity over 48 hrs post-dosing accounted for 12% of the administered dose in one male and 7% in one female. ... The radioactivity found in the carcasses and in other tissues accounted for 0.3% to 2.5% and <0.1% of the administered dose, respectively.
USEPA; Bensulide: Health Effects Division's Chapter for the Reregistration and Eligibility Decision Document. Washington, DC: USEPA, Off Pest Prog. p.17-18 (March 3, 1998)
Unchanged ring-labelled (14)-C bensulide could be detected in roots of lettuce plants after root application ... . A large part of the radioactive material in the leaves was evolved as radioactive carbon dioxide and some was present as labelled amino acids.
Weed Science Society of America. Herbicide Handbook. 5th ed. Champaign, Illinois: Weed Science Society of America, 1983., p. 49


