



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
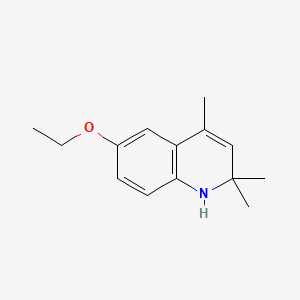

1. 1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline
2. 6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline
3. 6-etmdq
4. Santoquin
1. 91-53-2
2. 6-ethoxy-2,2,4-trimethyl-1,2-dihydroquinoline
3. Ethoxyquine
4. Santoquin
5. Santoquine
6. Niflex
7. Antioxidant Ec
8. Santoflex A
9. Santoflex Aw
10. Stop-scald
11. Dawe's Nutrigard
12. Nix-scald
13. Nocrack Aw
14. Quinol Ed
15. (-)-normetazocine
16. Permanax 103
17. Aries Antox
18. Niflex D
19. Antage Aw
20. Nocrac Aw
21. Alterungsschutzmittel Ec
22. Ethoxyquin [iso]
23. Ethoxychin
24. Amea 100
25. Quinoline, 6-ethoxy-1,2-dihydro-2,2,4-trimethyl-
26. Usaf B-24
27. Santoquine (van)
28. 1,2-dihydro-6-ethoxy-2,2,4-trimethylquinoline
29. 6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline
30. Santoflex
31. 6-ethoxy-2,2,4-trimethyl-1h-quinoline
32. Nsc-6795
33. Antox
34. 1,2-dihydro-2,2,4-trimethyl-6-ethoxyquinoline
35. 2,2,4-trimethyl-6-ethoxy-1,2-dihydroquinoline
36. Emq
37. 63301-91-7
38. Eq
39. Chebi:77323
40. Quinoline, 6-ethoxy-1,2-dihydro-2,2,4-trimethyl-, Homopolymer
41. 9t1410r4or
42. 6-(ethyloxy)-2,2,4-trimethyl-1,2-dihydroquinoline
43. Cas-91-53-2
44. Ncgc00016348-02
45. E324
46. Dsstox_cid_582
47. Dsstox_rid_75672
48. Dsstox_gsid_20582
49. Ethoxychin [czech]
50. Ethyl 2,2,4-trimethyl-1,2-dihydro-6-quinolinyl Ether
51. Caswell No. 427d
52. Ccris 2513
53. Hsdb 400
54. Einecs 202-075-7
55. 6-etmdq
56. Epa Pesticide Chemical Code 055501
57. Polyflex
58. Santoquineq
59. Unii-9t1410r4or
60. Ai3-17715
61. Prestwick_1064
62. Spectrum_001214
63. Ethoxyquin [mi]
64. Ethoxyquin [fcc]
65. Prestwick0_000765
66. Prestwick1_000765
67. Prestwick2_000765
68. Prestwick3_000765
69. Spectrum2_001384
70. Spectrum3_001423
71. Spectrum4_000404
72. Spectrum5_001530
73. Ethoxyquin [hsdb]
74. Ethoxyquin [mart.]
75. Schembl21601
76. Bspbio_000810
77. Bspbio_003126
78. Kbiogr_000668
79. Kbioss_001694
80. Mls001055488
81. Bidd:gt0822
82. Divk1c_000183
83. Spectrum1500998
84. Spbio_001368
85. Spbio_002749
86. Bpbio1_000892
87. Chembl172064
88. Dtxsid9020582
89. Hms500j05
90. Kbio1_000183
91. Kbio2_001694
92. Kbio2_004262
93. Kbio2_006830
94. Kbio3_002346
95. Nsc6795
96. Ninds_000183
97. Hms1570i12
98. Hms1923k03
99. Hms2097i12
100. Hms3714i12
101. 1,2,4-trimethyl-6-ethoxyquinoline
102. Hy-b1425
103. Nsc 6795
104. Zinc3872521
105. 6-ethoxy-1,2,4-trimethylquinoline
106. Tox21_110388
107. Tox21_201336
108. Tox21_300328
109. Ccg-39002
110. Ethoxyquin, >=75% (capillary Gc)
111. Mfcd00023883
112. S5369
113. Stk772149
114. Akos000121441
115. Cs-8200
116. Idi1_000183
117. Ncgc00016348-01
118. Ncgc00016348-03
119. Ncgc00016348-04
120. Ncgc00016348-05
121. Ncgc00016348-06
122. Ncgc00016348-07
123. Ncgc00016348-08
124. Ncgc00016348-09
125. Ncgc00016348-15
126. Ncgc00090792-01
127. Ncgc00090792-02
128. Ncgc00090792-03
129. Ncgc00090792-04
130. Ncgc00090792-05
131. Ncgc00254395-01
132. Ncgc00258888-01
133. Ac-11742
134. As-14741
135. Smr000686069
136. Db-057260
137. Ethoxyquin, Vetec(tm) Reagent Grade, 75%
138. Wln: T66 Bm Chj C1 C1 E1 Ho2
139. E 324
140. E0237
141. Ft-0621107
142. Ft-0673108
143. 2,4-trimethyl-6-ethoxy-1,2-dihydroquinoline
144. 6-ethoxy-2,4-trimethyl-1,2-dihydroquinoline
145. Ethoxyquin, Pestanal(r), Analytical Standard
146. 2,2, 4-trimethyl-6-ethoxy-1,2-dihydroquinoline
147. 6-ethoxy-1, 2-dihydro-2,2,4-trimethylquinoline
148. 6-ethoxy-1,2-dihydro-2,2,4-trimethyl-quinoline
149. 6-ethoxy-2,2,4-trimethyl-1, 2-dihydroquinoline
150. A843964
151. Sr-01000854319
152. J-010251
153. Q1657816
154. Sr-01000854319-2
155. W-100306
156. Brd-k56792340-001-05-8
157. 6-ethoxy-1,2-dihydro-2,2,4-trimethylquinoline, 9ci, 8ci
158. A10ae280-05f4-4846-acae-d565b0d263a2
159. Ethyl 2,2,4-trimethyl-1,2-dihydro-6-quinolinyl Ether #
160. 1,2-dihydro-2,2,4-trimethylquinolin-6-yl Ethyl Ether
161. Ethoxyquin. Short Expiry Date Due To Chemical Nature Of Component(s)
| Molecular Weight | 217.31 g/mol |
|---|---|
| Molecular Formula | C14H19NO |
| XLogP3 | 3.1 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 217.146664230 g/mol |
| Monoisotopic Mass | 217.146664230 g/mol |
| Topological Polar Surface Area | 21.3 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 283 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
(VET): Ethoxyquin is added to animal feeds at 0.015% as an antioxidant ... to help prevent encephalomalacia in growing chickens.
Booth, N.H., L.E. McDonald (eds.). Veterinary Pharmacology and Therapeutics. 5th ed. Ames, Iowa: Iowa State University Press, 1982., p. 1096
(VET): SANTOQUIN HAS BEEN SUCCESSFULLY USED WITH VITAMIN E IN TREATMENT OF WHITE MUSCLE DISEASE IN LAMBS.
Garner's Veterinary Toxicology. 3rd ed., rev. by E.G.C. Clarke and M.L. Clarke. Baltimore: Williams and Wilkins, 1967., p. 286
Vitamin E and also several synthetic antioxidants, such as ... ethoxyquin, have modified tumor induction by certain carcinogens in a number of target organs. However, inhibition was achieved at high doses at which induction of biotransformation enzymes also occurs with the synthetics and, therefore, inhibition may not be due solely to antioxidant effects.
Amdur, M.O., J. Doull, C.D. Klaasen (eds). Casarett and Doull's Toxicology. 4th ed. New York, NY: Pergamon Press, 1991., p. 152
3= MODERATELY TOXIC: PROBABLE ORAL LETHAL DOSE (HUMAN) 0.5-5 G/KG; BETWEEN 1 OZ & 1 PINT (OR 1 LB) FOR 70 KG PERSON (150 LB).
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-406
FEEDING TRIAL IN COWS WAS CONDUCTED TO DETERMINE WHETHER ETHOXYQUIN OR ITS RESIDUES ARE TRANSFERRED FROM FEED TO MILK WHEN ADDED TO COW FEED AT 0.015% OF DRY MATTER INTAKE. LESS THAN 7 UG ETHOXYQUIN/L WAS DETECTED IN MILK BY FLUORIMETRY & BY THIN-LAYER CHROMATOGRAPHY.
PMID:5669931 DUNKLEY WL ET AL; J DAIRY SCI 51 (8): 1215-8 (1968)
(14)C-ETHOXYQUIN WAS DISTRIBUTED THROUGHOUT MOST TISSUES & BLOOD AT 0.5 HR AFTER ADMIN TO RATS. HIGHEST RADIOACTIVITY THROUGHOUT EXPERIMENT WAS OBSERVED IN LIVER, KIDNEY, GI TRACT & ADIPOSE TISSUE. THERE WAS NO ACTIVITY IN CNS. OF DOSE INGESTED BY RAT 2.2 & 0.2% WERE FOUND IN LIVER AT 0.5 HR & 6 DAYS RESPECTIVELY FOLLOWING DOSE. HEPATIC PEAK IN RADIOACTIVITY WAS MEASURED AT 8 HR; & AFTER 6 DAYS 7.5% OF THIS LEVEL WAS STILL PRESENT IN LIVER. 6 DAYS AFTER ADMIN TO RATS RESIDUES OF ETHOXYQUIN & METABOLITES WERE PRESENT IN KIDNEY CORTEX, INTESTINES, LUNG, VARIOUS ADIPOSE TISSUES & BLOOD.
SKAARE JU, NAFSTAD I; ACTA PHARMACOL TOXICOL 44 (4): 303-7 (1979)
The compound is readily absorbed, metabolized, and excreted in urine and feces.
Gosselin, R.E., R.P. Smith, H.C. Hodge. Clinical Toxicology of Commercial Products. 5th ed. Baltimore: Williams and Wilkins, 1984., p. II-406
Ethoxyquin residue levels in the mouse tissue were determined by the HPLC-fluorometric detection method. Mice were given powdered feed containing 0, 0.125, and 0.5% ethoxyquin hydrochloric acid and the ethoxyquin residue levels in liver kidney, lung, and brain tissues were determined after 2, 4, 6, 10, and 14 wk (4 mice/group). The tissue samples were homogenized in 10 volumes (w/v) of acetonitrile-water (7:3 v/v) centrifuged and the supernatants were stored in a freezer for 2-3 hr or until the two layers separated; then the clear upper layers were analyzed. The mean ethoxyquin residue levels in the tissue ranged 0.84-4.58 ug ethoxyquin/g liver and 0.11-0.92 ug ethoxyquin/g brain. The relative weight of the liver (5.21-7.07% body weight) and the hepatic glutathione level (5.99-7.83 uM GSH/g tissue) of mice that received ethoxyquin were significantly higher than those of the controls (4.67-5.05% body weight and 4.30-5.78 uM GSH/g tissue, respectively). The mean hepatic mitochondrial glutathione levels of the higher ethoxyquin feeding group following dietary administration of ethoxyquin for 14 wk, was approximately twofold (1.68 nM GSH/mg protein) of both the control and the lower ethoxyquin feeding groups (0.83 and 0.74 nM GSH/mg protein, respectively.
PMID:2051496 Kim HL; J Toxicol Environ Health 33 (21): 229-36 (1991)
MAJOR METABOLIC REACTION WAS DEETHYLATION OF ETHOXYQUIN WHICH PRODUCED 6-HYDROXY-2,2,4-TRIMETHYL-1,2-DIHYDROQUINOLINE & 2,2,4-TRIMETHYL-6-QUINOLINE. OTHER REACTIONS WERE HYDROXYLATION TO FOUR DIFFERENT HYDROXYLATED METABOLITES & ONE DIHYDROXYLATED METABOLITE.
PMID:532214 SKAARE JU, SOLHEIM E; XENOBIOTICA 9 (11): 649-57 (1979)
An avg of 28 & 36% of dose of radioactivity was recovered in bile in 12 & 24 hr respectively following intragastric admin of (14)C-ethoxyquin to bile duct cannulated rats. Biliary radioactive substances included, in addn to unchanged ethoxyquin...8-hydroxyethoxyquin; hydroxylated 8-hydroxyethoxyquin; 6-ethoxy-2,2,4-trimethylquinolone; hydroxylated 6-ethoxy-2,2,4-trimethyl-8-quinolone; 6-ethoxy-2,4-dimethylquinoline; and 2,2,4-trimethyl-6-quinolone.
The Royal Society of Chemistry. Foreign Compound Metabolism in Mammals. Volume 6: A Review of the Literature Published during 1978 and 1979. London: The Royal Society of Chemistry, 1981., p. 297


