



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
0
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
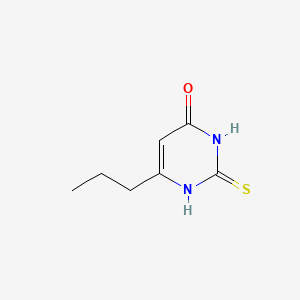

1. 6 Propyl 2 Thiouracil
2. 6-propyl-2-thiouracil
1. 51-52-5
2. 6-propyl-2-thiouracil
3. Propacil
4. Procasil
5. Prothiurone
6. Prothyran
7. Propycil
8. 2-mercapto-6-propylpyrimidin-4-ol
9. Prothiucil
10. Protiural
11. Thiuragyl
12. 6-n-propylthiouracil
13. Prothycil
14. Propyl-thiorist
15. Propyl-thyracil
16. Thyreostat Ii
17. Propythiouracil
18. Propilthiouracil
19. 6-propylthiouracil
20. Propyl-thiorit
21. 6-propyl-2-thioxo-2,3-dihydropyrimidin-4(1h)-one
22. Propylthiorit
23. 6-thio-4-propyluracil
24. 6-n-propyl-2-thiouracil
25. Propylthiouracile
26. Ptu (thyreostatic)
27. 4-propyl-2-thiouracil
28. Propiltiouracilo
29. Propylthiouracilum
30. 2-mercapto-6-propyl-4-pyrimidone
31. 2-mercapto-6-propylpyrimid-4-one
32. 2-thio-6-propyl-1,3-pyrimidin-4-one
33. 2-thio-4-oxo-6-propyl-1,3-pyrimidine
34. 6-propil-tiouracile
35. Uracil, 6-propyl-2-thio-
36. 6-propyl-2-sulfanylidene-1h-pyrimidin-4-one
37. Nsc 6498
38. 2-mercapto-4-hydroxy-6-n-propylpyrimidine
39. 2,3-dihydro-6-propyl-2-thioxo-4(1h)-pyrimidinone
40. 6-propyl-2-thio-2,4(1h,3h)pyrimidinedione
41. 500-50-5
42. 4(1h)-pyrimidinone, 2,3-dihydro-6-propyl-2-thioxo-
43. Mfcd00006041
44. Chebi:8502
45. 6-propyl-2 Thiouracil
46. Nsc-6498
47. Nsc-70461
48. Mls000028494
49. 721m9407iy
50. Cas-51-52-5
51. Ncgc00022715-03
52. Smr000058275
53. Propiltiouracile
54. Dsstox_cid_1209
55. Propiltiouracile [dcit]
56. 6-n-propylthiouracil;6-propyl-2-thiouracil;ptu
57. Dsstox_rid_76011
58. Dsstox_gsid_21209
59. Wln: T6mymvj Bus F3
60. 4-hydroxy-2-mercapto-6-propylpyrimidine
61. 6-propil-tiouracile [italian]
62. Propiltiouracilo [inn-spanish]
63. Propyl Thiouracil
64. Propylthiouracile [inn-french]
65. Propylthiouracilum [inn-latin]
66. 6-propyl-2-thio-2,3h)-pyrimidinedione
67. Ccris 544
68. Hsdb 3390
69. Propylthiouracil (tn)
70. Sr-05000001706
71. 4(1h)-pyrimidinone,3-dihydro-6-propyl-2-thioxo-
72. Einecs 200-103-2
73. 6-(n-propyl)-2-thiouracil
74. Ai3-25477
75. Unii-721m9407iy
76. 6-propyl-2-sulfanylpyrimidin-4-ol
77. Prestwick_810
78. 6-propyl-2-sulfanylidene-1,2,3,4-tetrahydropyrimidin-4-one
79. Propylthiouracil [usp:inn:ban:jan]
80. 6-propyl-2-thioxo-1h-pyrimidin-4-one
81. 6-propyl-2-sulfanylidene-2,3-dihydropyrimidin-4(1h)-one
82. Spectrum_000876
83. 2-mercapto-6-propylpyrimidin-4(3h)-one
84. Opera_id_530
85. 3cj
86. Prestwick0_000494
87. Prestwick1_000494
88. Prestwick2_000494
89. Prestwick3_000494
90. Spectrum2_001302
91. Spectrum3_001731
92. Spectrum4_000492
93. Spectrum5_001815
94. 6-propyl-2-thio-2,4(1h,3h)-pyrimidinedione
95. 2-thio-4-hydroxy-6-n-propyl-pyrimidine
96. Chembl1518
97. Schembl41239
98. Bspbio_000387
99. Bspbio_003402
100. Kbiogr_001003
101. Kbioss_001356
102. Propylthiouracil [mi]
103. Mls002303010
104. Mls006011901
105. Divk1c_000268
106. Propylthiouracil [inn]
107. Propylthiouracil [jan]
108. Spectrum1500515
109. Spbio_001363
110. Spbio_002308
111. Propylthiouracil [hsdb]
112. Propylthiouracil [iarc]
113. 6-propyl-2-thioxo-2, 3-dihydropyrimidin-4(1h)-one
114. Bpbio1_000427
115. Gtpl6650
116. Propylthiouracil [vandf]
117. Dtxsid5021209
118. Propylthiouracil [mart.]
119. Schembl17375339
120. Hms500n10
121. Kbio1_000268
122. Kbio2_001356
123. Kbio2_003924
124. Kbio2_006492
125. Kbio3_002622
126. Nsc6498
127. Propylthiouracil [usp-rs]
128. Propylthiouracil [who-dd]
129. Propylthiouracil [who-ip]
130. Ninds_000268
131. Hms1569d09
132. Hms1766d22
133. Hms1920l22
134. Hms2092e03
135. Hms2096d09
136. Hms2230b22
137. Hms3259m04
138. Hms3371d17
139. Hms3655j07
140. Hms3713d09
141. Pharmakon1600-01500515
142. Bcp22165
143. Hy-b0346
144. Nsc70461
145. Propylthiouracil (jp17/usp/inn)
146. Zinc4640636
147. Tox21_110882
148. Tox21_201741
149. Tox21_300280
150. 2-mercapto-6-propyl-pyrimidin-4-ol
151. Ac8761
152. Bdbm50133597
153. Ccg-39240
154. Nsc 70461
155. Nsc757302
156. S1988
157. Stl102645
158. Propylthiouracil [ep Impurity]
159. Propylthiouracil [orange Book]
160. 6-propyl-2-sulfanyl-4-pyrimidinol #
161. Akos000120319
162. Akos001053246
163. Akos015892537
164. Tox21_110882_1
165. 2-thio-4-hydroxy-6-n-propylpyrimidine
166. Bs-3928
167. Db00550
168. Nc00533
169. Nsc-757302
170. Propylthiouracil [ep Monograph]
171. Ps-3436
172. 4-hydroxy-6-n-propylpyrimidine-2-thiol
173. Idi1_000268
174. Propylthiouracil [usp Monograph]
175. 6-propyl-2-thiouracil, Enzyme Inhibitor
176. Ncgc00016229-01
177. Ncgc00016229-02
178. Ncgc00016229-03
179. Ncgc00016229-04
180. Ncgc00016229-05
181. Ncgc00016229-06
182. Ncgc00016229-07
183. Ncgc00016229-08
184. Ncgc00016229-09
185. Ncgc00016229-10
186. Ncgc00016229-11
187. Ncgc00016229-13
188. Ncgc00016229-14
189. Ncgc00090881-01
190. Ncgc00090881-02
191. Ncgc00090881-03
192. Ncgc00178089-01
193. Ncgc00178089-02
194. Ncgc00183321-01
195. Ncgc00254180-01
196. Ncgc00259290-01
197. 2-mercapto-4-hydroxy-6-propyl Pyrimidine
198. Ac-10795
199. Propylthiouracil / 6-propyl-2-thiouracil
200. Smr003317355
201. Sy038617
202. Sbi-0051497.p003
203. Bb 0242498
204. Ft-0621285
205. Ft-0695546
206. P0533
207. Sw196944-3
208. C07569
209. D00562
210. 6-propyl-2-sulfanyl-3,4-dihydropyrimidin-4-one
211. 6-propyl-2-thiouracil, Purum, >=98.0% (t)
212. Ab00052082_05
213. Ab00052082_06
214. Q377342
215. Sr-05000001706-1
216. Sr-05000001706-2
217. Sr-05000001706-3
218. W-105881
219. 6-propyl-2-thioxo-2,3-dihydro-1h-pyrimidin-4 -one
220. Brd-k48168960-001-04-4
221. Brd-k48168960-001-05-1
222. Brd-k48168960-001-08-5
223. Z56922173
224. F1967-1318
225. F2199-0035
226. F3097-4245
227. 4(1h)-pyrimidinone,6-butyl-2,3-dihydro-2-thioxo-
228. 6-propyl-2-thiouracil, Vetranal(tm), Analytical Standard
229. Propylthiouracil, European Pharmacopoeia (ep) Reference Standard
230. Propylthiouracil, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 170.23 g/mol |
|---|---|
| Molecular Formula | C7H10N2OS |
| XLogP3 | 0.8 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 2 |
| Exact Mass | 170.05138412 g/mol |
| Monoisotopic Mass | 170.05138412 g/mol |
| Topological Polar Surface Area | 73.2 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 223 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Propylthiouracil |
| PubMed Health | Propylthiouracil (By mouth) |
| Drug Classes | Antithyroid Agent |
| Drug Label | Propylthiouracil, USP is one of the thiocarbamide compounds. It is a white, crystalline substance that has a bitter taste and is very slightly soluble in water. Propylthiouracil is an antithyroid drug administered orally. The structural formula is:Ea... |
| Active Ingredient | Propylthiouracil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Dava Pharms; Actavis Elizabeth |
| 2 of 2 | |
|---|---|
| Drug Name | Propylthiouracil |
| PubMed Health | Propylthiouracil (By mouth) |
| Drug Classes | Antithyroid Agent |
| Drug Label | Propylthiouracil, USP is one of the thiocarbamide compounds. It is a white, crystalline substance that has a bitter taste and is very slightly soluble in water. Propylthiouracil is an antithyroid drug administered orally. The structural formula is:Ea... |
| Active Ingredient | Propylthiouracil |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 50mg |
| Market Status | Prescription |
| Company | Dava Pharms; Actavis Elizabeth |
Mesh Heading: Antimetabolites, Antithyroid Agents
National Library of Medicine, SIS; ChemIDplus Record for Propylthiouracil (51-52-5). Available from, as of April 17, 2006: https://chem.sis.nlm.nih.gov/chemidplus/chemidlite.jsp
... Propylthiouracil /is/ indicated in the treatment of hyperthyroidism, including prior to surgery or radiotherapy, and as adjuncts in the treatment of thyrotoxicosis or thyroid storm. Propylthiouracil may be preferred over methimazole for use in thyroid storm, since propylthiouracil inhibits peripheral conversion of thyroxine (T4) to triiodothyronine (T3).
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 450
EXPTL USE: Paradoxically propylthiouracil has been shown to reverse histological changes of alcoholic hepatitis in rat and has been proposed as possible treatment for this condition in man.
Miller, R. R., and D. J. Greenblatt. Handbook of Drug Therapy. New York: Elsevier North Holland, 1979., p. 917
EXPTL USE: Twelve-day pretreatment with PTU prevented the tylenol-induced increase in transaminase activities. Increase in hepatic reduced glutathione levels and prevention of inflammatory response to necrotic liver tissue appeared to be mechanism in protective action of hypothyroidism.
PMID:7350016 LINSCHEER ET AL; GASTROENTEROLOGY 78 (1): 100 (1980)
For more Therapeutic Uses (Complete) data for PROPYL THIOURACIL (14 total), please visit the HSDB record page.
Although reported much less frequently, severe adverse effects, including inhibition of myelopoiesis with resultant agranulocytosis, granulocytopenia, and thrombocytopenia; aplastic anemia; drug fever; lupus-like syndrome (including splenomegaly); severe hepatic reactions (including encephalopathy, fulminant hepatic necrosis, and death); periarteritis; and hypoprothrombinemia and bleeding, have been reported to occur in some patients receiving propylthiouracil. Nephritis and interstitial pneumonitis have also been reported. Cutaneous vasculitis, which may manifest as purpuric and/or bullous hemorrhagic lesions or erythema nodosum, and possibly may progress to necrotic ulcerations, and polymyositis also have occurred.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3192
Agranulocytosis is potentially the most serious adverse effect of propylthiouracil, and most cases of agranulocytosis appear to occur within the first 2 months of therapy, but rarely may occur after 4 months of therapy. The risk of propylthiouracil-induced agranulocytosis appears to be substantially increased in patients older than 40 years of age compared with younger patients, but, unlike methimazole, an association with dosage has not been established. Although the mechanism(s) of propylthiouracil-induced agranulocytosis has not been determined, antigranulocyte antibodies have been reported in some patients with thioamide-induced agranulocytosis; a direct toxic effect of these drugs on bone marrow has not been excluded as an additional possible cause.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3192
Propylthiouracil crosses the placenta and may cause fetal harm when administered to pregnant women; the drug can induce goiter and hypothyroidism (cretinism) in the developing fetus. If the drug is used during pregnancy for the management of hyperthyroidism, the manufacturer states that careful dosage adjustment, using a sufficient but not excessive dosage of propylthiouracil, is necessary. The manufacturer states that because thyroid dysfunction diminishes in many women as pregnancy proceeds, a reduction in dosage may be possible, and, in some patients, propylthiouracil can be discontinued 2 or 3 weeks before delivery. If propylthiouracil is used during pregnancy, or if a woman becomes pregnant while receiving the drug, she should be advised of the potential hazard to the fetus.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3192
... Disagreement about therapy of thyrotoxicosis during pregnancy. Antithyroid drugs cross placenta and can cause fetal hypothyroidism and goiter. ... There are 3 choices of therapy, each with its advocates: minimal doses of antithyroid drugs, full doses ... with thyroid hormone supplementation, or surgery. /Antithyroid drugs/
Gilman, A.G., T.W. Rall, A.S. Nies and P. Taylor (eds.). Goodman and Gilman's The Pharmacological Basis of Therapeutics. 8th ed. New York, NY. Pergamon Press, 1990., p. 1375
For more Drug Warnings (Complete) data for PROPYL THIOURACIL (20 total), please visit the HSDB record page.
Used to manage hyperthyroidism which is due to an overactive thyroid gland (Grave's disease).
Propylthiouracil is a thiourea antithyroid agent. Grave's disease is the most common cause of hyperthyroidism. It is an autoimmune disease where an individual's own antibodies attach to thyroid stimulating hormone receptors within cells of the thyroid gland and then trigger overproduction of thyroid hormone. The two thyroid hormones manufactured by the thyroid gland, thyroxine (T4) and triiodothyronine (T3), are formed by combining iodine and a protein called thyroglobulin with the assistance of an enzyme called peroxidase. PTU inhibits iodine and peroxidase from their normal interactions with thyroglobulin to form T4 and T3. This action decreases thyroid hormone production. PTU also interferes with the conversion of T4 to T3, and, since T3 is more potent than T4, this also reduces the activity of thyroid hormones. The actions and use of propylthiouracil are similar to those of methimazole.
Antimetabolites
Drugs that are chemically similar to naturally occurring metabolites, but differ enough to interfere with normal metabolic pathways. (From AMA Drug Evaluations Annual, 1994, p2033) (See all compounds classified as Antimetabolites.)
Antithyroid Agents
Agents that are used to treat hyperthyroidism by reducing the excessive production of thyroid hormones. (See all compounds classified as Antithyroid Agents.)
H03BA02
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
H - Systemic hormonal preparations, excl. sex hormones and insulins
H03 - Thyroid therapy
H03B - Antithyroid preparations
H03BA - Thiouracils
H03BA02 - Propylthiouracil
Absorption
Well absorbed following oral administration.
Route of Elimination
Propylthiouracil is readily absorbed and is extensively metabolized. Approximately 35% of the drug is excreted in the urine, in intact and conjugated forms, within 24 hours.
Elimination: Less than 1% is excreted in the urine unchanged. Total body clearance is approximately 7 L/hr. In dialysis: Elimination and pharmacokinetics are not significantly altered in hemodialysis. In one patient undergoing hemodialysis, 5% of a 200 mg oral dose was removed by 3 hours of hemodialysis; elimination rate was not significantly altered. Peak serum concentration was decreased (from 7.9 to 4.9 ug/mL), although it remained within an approximate therapeutic range.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 450
Although distribution of propylthiouracil into human body tissues and fluids has not been fully characterized, the drug appears to be concentrated in the thyroid gland. Propylthiouracil readily crosses the placenta. Propylthiouracil is distributed into milk; however, some studies indicate that the extent of distribution is only about 0.007-0.077% of a single dose.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3192
Propylthiouracil is rapidly and readily absorbed from the GI tract following oral administration with peak plasma concentrations of about 6-9 mcg/mL occurring within 1-1.5 hours after a single dose of 200-400 mg. In one study in which the drug was administered orally and IV, about 75% of the oral dose was absorbed. Plasma concentrations of the drug do not appear to correlate with the therapeutic effects.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3192
Time to peak effect: 17 weeks (average) to normalize serum T3 and T4 concentrations with use of 300 mg/day.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 450
For more Absorption, Distribution and Excretion (Complete) data for PROPYL THIOURACIL (19 total), please visit the HSDB record page.
Propylthiouracil was concentrated by thyroid gland, and four sulfur-35 compounds were demonstrated by TLC in both rat and man: unchanged propylthiouracil, (35)-sulfate, unknown propylthiouracil metabolite and protein-bound sulfur-35...
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V7 73 (1974)
Biotransformation: Primarily undergoes glucuronidation. Approximately 33% of an orally administered dose is metabolized by a first-pass effect.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 450
Presence of more than one glucuronide conjugate of propylthiouracil ought to be expected, as it has four functional groups, each capable of conjugation with glucuronic acid ...
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 307
Although the exact metabolic fate of propylthiouracil has not been fully established, the drug is rapidly metabolized to its glucuronide conjugate and other minor metabolites and requires frequent administration to maintain its antithyroid effect. The drug and its metabolites are excreted in urine, with about 35% of a dose excreted within 24 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3192
For more Metabolism/Metabolites (Complete) data for PROPYL THIOURACIL (8 total), please visit the HSDB record page.
2 hours
The elimination half-life of propylthiouracil has generally been reported to be about 1-2 hours.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3192
The plasma half-life of propylthiouracil .../is/ 1 to 2 hours.
Haddad, L.M. (Ed). Clinical Management of Poisoning and Drug Overdose 3rd Edition. Saunders, Philadelphia, PA. 1998., p. 1141
/After GI absorption/ plasma half-lives of 2.5 hr (human) and 4.8 hr (rat) have been reported ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V7 72 (1974)
The half-life of propylthiouracil in plasma is about 75 min ...
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1581
Propylthiouracil is rapidly absorbed from /orally/ dosed tablets in man, yielding max plasma levels at 60-120 min, and biological t/2 of about 60 min in euthyroid subjects.
The Chemical Society. Foreign Compound Metabolism in Mammals Volume 3. London: The Chemical Society, 1975., p. 163
Propylthiouracil binds to thyroid peroxidase and thereby inhibits the conversion of iodide to iodine. Thyroid peroxidase normally converts iodide to iodine (via hydrogen peroxide as a cofactor) and also catalyzes the incorporation of the resulting iodide molecule onto both the 3 and/or 5 positions of the phenol rings of tyrosines found in thyroglobulin. Thyroglobulin is degraded to produce thyroxine (T4) and tri-iodothyronine (T3), which are the main hormones produced by the thyroid gland. Therefore propylthiouracil effectively inhibits the production of new thyroid hormones.
Propylthiouracil inhibits the synthesis of thyroid hormones by interfering with the incorporation of iodine into tyrosyl residues of thyroglobulin; the drug also inhibits the coupling of these iodotyrosyl residues to form iodothyronine. Although the exact mechanism(s) has not been fully elucidated, propylthiouracil may interfere with the oxidation of iodide ion and iodotyrosyl groups. Based on limited evidence it appears that the coupling reaction is more sensitive to antithyroid agents than the iodination reaction. Propylthiouracil does not inhibit the action of thyroid hormones already formed and present in the thyroid gland or circulation nor does the drug interfere with the effectiveness of exogenously administered thyroid hormones. Patients whose thyroid gland contains relatively high concentration of iodine (e.g., from prior ingestion or from administration during diagnostic radiologic procedures) may respond relatively slowly to antithyroid agents. Unlike methimazole, propylthiouracil inhibits the peripheral deiodination of thyroxine to triiodothyronine. Although the importance of this inhibition has not been established, propylthiouracil has a theoretical advantage compared with methimazole or carbimazole in patients with thyrotoxic crisis, since a decreased rate of conversion of circulating thyroxine to triiodothyronine may be clinically beneficial in these patients.
McEvoy, G.K. (ed.). American Hospital Formulary Service. AHFS Drug Information. American Society of Health-System Pharmacists, Bethesda, MD. 2006., p. 3192
The thionamide /propylthiouracil/ inhibit organification of iodide and the coupling of iodotyrosines to form hormonally active iodothyronines.
Haddad, L.M. (Ed). Clinical Management of Poisoning and Drug Overdose 3rd Edition. Saunders, Philadelphia, PA. 1998., p. 1141
Inhibit synthesis of thyroid hormone within the thyroid gland by serving as substrates for thyroid peroxidase, which catalyzes the incorporation of oxidized iodide into tyrosine residues in thyroglobulin molecules and couples iodotyrosines. This diverts iodine from the synthesis of thyroid hormones. Antithyroid agents do not interfere with the actions of exogenous thyroid hormone or inhibit the release of thyroid hormones. Therefore, stores of thyroid hormones must be depleted before clinical effects will be apparent. Antithyroid agents may also have moderating effects on the underlying immunologic abnormalities, in hyperthyroidism due to Graves' disease (toxic-diffuse goiter), but evidence on this point reported to date is inconclusive.
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006., p. 450
Type I 5'-deiodinase (D1) is inhibited by ... the antithyroid drug propylthiouracil.
Hardman, J.G., L.E. Limbird, P.B., A.G. Gilman. Goodman and Gilman's The Pharmacological Basis of Therapeutics. 10th ed. New York, NY: McGraw-Hill, 2001., p. 1567
For more Mechanism of Action (Complete) data for PROPYL THIOURACIL (9 total), please visit the HSDB record page.






