



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
0
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
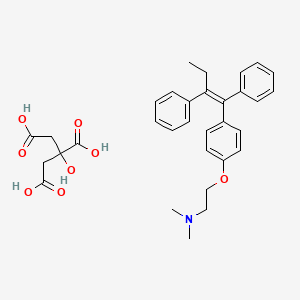

1. Citrate, Tamoxifen
2. Ici 46,474
3. Ici 46474
4. Ici 47699
5. Ici-46,474
6. Ici-46474
7. Ici-47699
8. Ici46,474
9. Ici46474
10. Ici47699
11. Nolvadex
12. Novaldex
13. Soltamox
14. Tamoxifen
15. Tomaxithen
16. Zitazonium
1. 54965-24-1
2. Zitazonium
3. Istubal
4. Kessar
5. Zemide
6. Tamoxifen (citrate)
7. Tamoxifen Citrate Salt
8. Nourytam
9. Tamofene
10. Tomaxasta
11. Ici 46474
12. Ici 46,474
13. Ici 46474 Citrate
14. Ici-46474
15. Nsc-180973
16. Nsc-757345
17. Tamoxifen Citrate (nolvadex)
18. 54965-24-1 (citrate)
19. 7frv7310n6
20. I.c.i.46474 Citrate
21. Nsc180973
22. Ncgc00024928-06
23. Cas-54965-24-1
24. Dsstox_cid_1301
25. (z)-2-(p-(1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethylethylamine Citrate (1:1)
26. Dsstox_rid_76068
27. Dsstox_gsid_21301
28. Ethanamine, 2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethyl-, (z)-,2-hydroxy-1,2,3-propanetricarboxylate (1:1)
29. Caditam
30. Farmifeno
31. Ginarsan
32. Jenoxifen
33. Ledertam
34. Nourytan
35. Noxitem
36. Oncotam
37. Tafoxen
38. Tamofen
39. Tamoxasta
40. Terimon
41. Zynoplex
42. Emblon
43. Genox
44. Nolgen
45. Noltam
46. Tamax
47. Tamoxifencitrate
48. Tamox-puren
49. (z)-2-(4-(1,2-diphenylbut-1-en-1-yl)phenoxy)-n,n-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate
50. 2-[4-[(z)-1,2-diphenylbut-1-enyl]phenoxy]-n,n-dimethylethanamine;2-hydroxypropane-1,2,3-tricarboxylic Acid
51. Ethanamine, 2-(4-(1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethyl, (z)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
52. Smr000677949
53. Z-tamoxifen Citrate
54. Sr-01000075523
55. Unii-7frv7310n6
56. Ccris 6718
57. Tamoxifeni Citras
58. Nolvadex (tn)
59. Soltamox (tn)
60. 2-{4-[(1z)-1,2-diphenylbut-1-en-1-yl]phenoxy}-n,n-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate (salt)
61. Prestwick_458
62. Einecs 259-415-2
63. Tamoxifen Citrate [usan:usp:jan]
64. Mfcd00058321
65. Tamoxifen (nolvadex)
66. Tamoxifen Citrate,(s)
67. Tamoxifen, Citrate Salt
68. Lopac-t-9262
69. Tamoxifen Z-isomer Citrate
70. Chembl786
71. Schembl6365
72. Mls001055370
73. Mls002154210
74. Spectrum1500557
75. Nolvadex (tn) (astrazeneca)
76. Tamoxifen Citrate [mi]
77. Tamoxifen Citrate [jan]
78. Dtxsid8021301
79. Tamoxifen Citrate (jp17/usp)
80. Tamoxifen Citrate [usan]
81. Hms500m20
82. Tamoxifen Citrate Salt, >=99%
83. Tamoxifen Citrate [vandf]
84. Tamoxifen Citrate [mart.]
85. Hms1568m14
86. Hms1921c13
87. Hms2092k17
88. Hms2095m14
89. Hms2232e08
90. Hms3263b08
91. Hms3675p04
92. Hms3712m14
93. Pharmakon1600-01500557
94. Tamoxifen Citrate [usp-rs]
95. Tamoxifen Citrate [who-dd]
96. Tamoxifen Citrate [who-ip]
97. Ex-a5777
98. Hydroxypropane-1,2,3-tricarboxylate
99. Tox21_110938
100. Tox21_202070
101. Tox21_300274
102. Tox21_501203
103. Ccg-39629
104. Nsc757345
105. S1238
106. S1972
107. Akos005111126
108. Tox21_110938_1
109. Cs-1852
110. Ks-5046
111. Lp01203
112. Tamoxifen Citrate [orange Book]
113. Tamoxifen Citrate [ep Monograph]
114. Ncgc00016206-01
115. Ncgc00016206-02
116. Ncgc00016206-03
117. Ncgc00016206-04
118. Ncgc00016206-05
119. Ncgc00016206-06
120. Ncgc00024928-02
121. Ncgc00024928-23
122. Ncgc00094450-01
123. Ncgc00094450-02
124. Ncgc00094450-03
125. Ncgc00094450-04
126. Ncgc00254000-01
127. Ncgc00259619-01
128. Ncgc00261888-01
129. Tamoxifen Citrate [usp Monograph]
130. Tamoxifeni Citras [who-ip Latin]
131. Hy-13757
132. Bcp0726000223
133. Tamoxifen Citrate - Cas 54965-24-1
134. Eu-0101203
135. Sw219368-1
136. T2510
137. (z)-2-(4-(1,2-diphenylbut-1-enyl)phenoxy)
138. A19582
139. D00966
140. T 9262
141. 965t241
142. Q-201776
143. Sr-01000075523-1
144. Sr-01000075523-3
145. Sr-01000075523-5
146. Sr-01000075523-6
147. Q27108378
148. Sr-01000075523-10
149. (z)-1-(4-dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene Citrate
150. Tamoxifen Citrate, European Pharmacopoeia (ep) Reference Standard
151. (z)-2-(4-(1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethylethanamine,citrate
152. (z)-2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethylethanamine Citrate
153. Tamoxifen Citrate, United States Pharmacopeia (usp) Reference Standard
154. (z)-2-[ P-(1,2-diphenyl-1-butenyl)phenoxy]- N, N-dimethylethylamine Citrate (1:1)
155. (z)-2-[4-(1,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethyl-ethanamine Cirtrate (1:1)
156. Tamoxifen Citrate For Performance Test, European Pharmacopoeia (ep) Reference Standard
157. (2-{4-[1,2-diphenylbut-1-en-1-yl]phenoxy}ethyl)dimethylamine; 2-hydroxypropane-1,2,3-tricarboxylic Acid
158. (z)-(2-(4-(1,2-diphenylbut-1-enyl)phenoxy)ethyl)dimethylammonium Dihydrogen 2-hydroxypropane-1,2,3-tricarboxylate
159. (z)-2-(4-(1,2-diphenylbut-1-enyl)phenoxy)-n,n-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate
160. {2-[4-(1,2-diphenyl-1-buten-1-yl)phenoxy]ethyl}dimethylamine 2-hydroxy-1,2,3-propanetricarboxylate Salt
161. Ethanamine, 2-(4-((1z)-1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethyl-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
162. Ethanamine,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethyl-, (z)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
| Molecular Weight | 563.6 g/mol |
|---|---|
| Molecular Formula | C32H37NO8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 13 |
| Exact Mass | 563.25191714 g/mol |
| Monoisotopic Mass | 563.25191714 g/mol |
| Topological Polar Surface Area | 145 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 690 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Tamoxifen citrate |
| Drug Label | NOLVADEX (tamoxifen citrate) Tablets, a nonsteroidal antiestrogen, are for oral administration. NOLVADEX Tablets are available as:... |
| Active Ingredient | Tamoxifen citrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Teva; Actavis Labs Fl; Apotex; Teva Pharms; Mylan |
| 2 of 2 | |
|---|---|
| Drug Name | Tamoxifen citrate |
| Drug Label | NOLVADEX (tamoxifen citrate) Tablets, a nonsteroidal antiestrogen, are for oral administration. NOLVADEX Tablets are available as:... |
| Active Ingredient | Tamoxifen citrate |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | eq 20mg base; eq 10mg base |
| Market Status | Prescription |
| Company | Teva; Actavis Labs Fl; Apotex; Teva Pharms; Mylan |
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
Estrogen Antagonists
Compounds which inhibit or antagonize the action or biosynthesis of estrogenic compounds. (See all compounds classified as Estrogen Antagonists.)
Selective Estrogen Receptor Modulators
A structurally diverse group of compounds distinguished from ESTROGENS by their ability to bind and activate ESTROGEN RECEPTORS but act as either an agonist or antagonist depending on the tissue type and hormonal milieu. They are classified as either first generation because they demonstrate estrogen agonist properties in the ENDOMETRIUM or second generation based on their patterns of tissue specificity. (Horm Res 1997;48:155-63) (See all compounds classified as Selective Estrogen Receptor Modulators.)








