



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
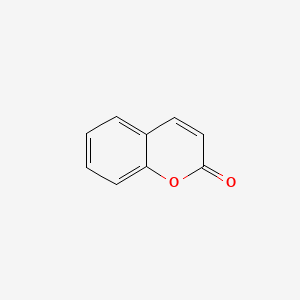

1. 1,2-benzopyrone
2. 5,6-benzo-alpha-pyrone
1. 91-64-5
2. 2h-chromen-2-one
3. 2h-1-benzopyran-2-one
4. 1,2-benzopyrone
5. Cumarin
6. Chromen-2-one
7. Rattex
8. Tonka Bean Camphor
9. Coumarinic Anhydride
10. Coumarine
11. Cis-o-coumarinic Acid Lactone
12. O-hydroxycinnamic Acid Lactone
13. Coumarinic Lactone
14. Benzo-alpha-pyrone
15. O-hydroxycinnamic Lactone
16. 2-oxo-1,2-benzopyran
17. Kumarin
18. 2h-benzo(b)pyran-2-one
19. Benzo-a-pyrone
20. Kumarin [czech]
21. 2h-1-benzopyran, 2-oxo-
22. 5,6-benzo-2-pyrone
23. 5,6-benzo-alpha-pyrone
24. 2h-benzo[b]pyran-2-one
25. Caswell No. 259c
26. O-coumaric Acid Lactone
27. O-hydroxyzimtsaure-lacton
28. Nci C07103
29. Cis-o-coumaric Acid Anhydride
30. Chromenone
31. Coumarinum
32. 103802-83-1
33. O-hydroxyzimtsaure-lacton [german]
34. Nsc 8774
35. 2-propenoic Acid, 3-(2-hydroxyphenyl)-, Delta-lactone
36. Epa Pesticide Chemical Code 127301
37. Brn 0383644
38. Benzo-.alpha.-pyrone
39. Cinnamic Acid, O-hydroxy-, Delta-lactone
40. Chebi:28794
41. Ai3-00753
42. Nsc-8774
43. O-hydroxycinnamic Acid Delta-lactone
44. 3-(2-hydroxyphenyl)-2-propenoic Delta-lactone
45. A4vz22k1wt
46. Chembl6466
47. Nci-c07103
48. 2-propenoic Acid, 3-(2-hydroxyphenyl)-delta-lactone
49. Mls000028741
50. Dtxsid7020348
51. Nsc8774
52. Ncgc00091502-01
53. Coumarin, >=98%
54. Smr000059040
55. 1-benzopyran-2-one
56. Dsstox_cid_348
57. 2h-chromene-2-one
58. Dsstox_rid_75529
59. Dsstox_gsid_20348
60. 2-propenoic Acid, 3-(2-hydroxyphenyl)-, D-lactone
61. 2-propenoic Acid, 3-(2-hydroxyphenyl)-, .delta.-lactone
62. Benzopyranone
63. Cas-91-64-5
64. Coumarin [nf]
65. Cou
66. Ccris 181
67. 2h-benzopyran-2-one
68. Hsdb 1623
69. Sr-01000721887
70. 2-oxo-2h-1-benzopyran
71. Einecs 202-086-7
72. Mfcd00006850
73. Unii-a4vz22k1wt
74. Chromen-one
75. A Coumarin
76. Coumarin-
77. D-lactone
78. Alpha-benzopyrone
79. Benzopyrylium Olate
80. Coumarin (dcf)
81. 1, 2-benzopyrone
82. A 1,2-benzopyrone
83. Venalot Mono (tn)
84. Coumarin (prohibited)
85. Spectrum_001336
86. St023509
87. Coumarin [hsdb]
88. Coumarin [iarc]
89. Coumarin [inci]
90. Opera_id_268
91. 2h-chromen-2-one #
92. 3-(2-hydroxyphenyl)-
93. Coumarin [mi]
94. 2h-1-benzopyran-2-on
95. Spectrum2_000303
96. Spectrum3_001772
97. Spectrum4_001818
98. Spectrum5_000555
99. Coumarinum [hpus]
100. Coumarin [mart.]
101. Bmse000077
102. Coumarin [who-dd]
103. Epitope Id:114082
104. Ec 202-086-7
105. Schembl6252
106. Wln: T66 Bovj
107. Bspbio_003263
108. Kbiogr_002460
109. Kbioss_001816
110. 5-17-10-00143 (beilstein Handbook Reference)
111. Mls001148422
112. Mls002454395
113. {2h-benzo[b]pyran-2-one}
114. Bidd:er0667
115. Spectrum1400208
116. Spbio_000266
117. Cinnamic Acid, .delta.-lactone
118. Coumarin, >=99% (hplc)
119. Bdbm12342
120. Kbio2_001816
121. Kbio2_004384
122. Kbio2_006952
123. Kbio3_002764
124. Zinc74709
125. Glxc-19130
126. Hms1923m11
127. Hms2091e19
128. Hms2232h18
129. Hms3369l08
130. Hms3652b05
131. Hms3885d09
132. Pharmakon1600-01400208
133. Amy37188
134. Hy-n0709
135. Tox21_111141
136. Tox21_202427
137. Tox21_300057
138. Ccg-38580
139. Nsc755852
140. S4170
141. Stk066167
142. Coumarin (prohibited) [fhfi]
143. Akos000120175
144. Tox21_111141_1
145. 2h-chromen-2-one (acd/name 4.0)
146. Cr-0048
147. Cs-8148
148. Db04665
149. Nsc-755852
150. Sdccgmls-0066912.p001
151. Ncgc00091502-02
152. Ncgc00091502-03
153. Ncgc00091502-04
154. Ncgc00091502-05
155. Ncgc00091502-06
156. Ncgc00091502-07
157. Ncgc00091502-08
158. Ncgc00091502-09
159. Ncgc00091502-11
160. Ncgc00091502-16
161. Ncgc00254092-01
162. Ncgc00259976-01
163. Coumarin 1000 Microg/ml In Acetonitrile
164. Nci60_041938
165. Sbi-0061760.p002
166. Db-057267
167. Cinnamic Acid, O-hydroxy-, .delta.-lactone
168. Coumarin, Vetec(tm) Reagent Grade, >=99%
169. Ft-0606287
170. Ft-0665197
171. N1707
172. Sw220278-1
173. A14543
174. Bim-0061760.0001
175. C05851
176. D07751
177. D81844
178. Ab00375898_11
179. Ab00375898_12
180. Coumarin, Primary Pharmaceutical Reference Standard
181. Q111812
182. Cu-01000013121-2
183. Q-100890
184. Sr-01000721887-2
185. Sr-01000721887-3
186. Brd-k23913458-001-02-5
187. Brd-k23913458-001-13-2
188. Coumarin, Certified Reference Material, Tracecert(r)
189. Z57169486
190. Coumarin, European Pharmacopoeia (ep) Reference Standard
191. F3096-1712
192. Coumarin (constituent Of Cinnamomum Cassia Bark) [dsc]
193. Coumarin (constituent Of Cinnamomum Verum Bark) [dsc]
194. D3e956c4-9541-4f57-9435-7d915c38e19e
195. 2h-1-benzopyran-2-one;coumarin;2h-chromen-2-one;coumarin ;coumarin (2h-1-benzopyran-2-one) (chromen-2-one);2h-1-benzopyran-2-one Coumarin 2h-chromen-2-one Coumarin Coumarin (2h-1-benzopyran-2-one) (chromen-2-one)
| Molecular Weight | 146.14 g/mol |
|---|---|
| Molecular Formula | C9H6O2 |
| XLogP3 | 1.4 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 146.036779430 g/mol |
| Monoisotopic Mass | 146.036779430 g/mol |
| Topological Polar Surface Area | 26.3 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 196 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
A species difference has been reported for the excretion of an oral dose of (14)C-coumarin. Within 4 days rats excreted 47% of the label in the urine and 39% in the feces, whereas rabbits excreted 92% in the urine and negligible amount in the feces.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 87
Female rabbits dosed orally with 50 mg/kg of 3-14C-coumarin excreted over 80% of the label in the urine in 24 hours. No label was found in the expired air and only a small amount in the feces.
Joint Expert Committee on Food Additives; WHO Food Additive Series #16, Coumarin. Available from, as of March 21, 2003: https://www.inchem.org/documents/jecfa/jecmono/v16je10.htm
The reason for the considerable fecal excretion of (14)C /after oral administration of (14)C-coumarin/ in rat... may represent unabsorbed material.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 87
Twenty-four hr after an IP dose to rats of... (14)C-coumarin, 38% had been excreted in the urine, 13% in the feces, 30% was excreted in the air as (14)C-carbon dioxide and 9% of the remainder was mainly present in the cecum.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 2: A Review of the Literature Published Between 1970 and 1971. London: The Chemical Society, 1972., p. 151
For more Absorption, Distribution and Excretion (Complete) data for COUMARIN (14 total), please visit the HSDB record page.
...Recombinant human and rat CYP1A forms and recombinant human CYP2E1 readily catalyzed CE /coumarin-3,4-epoxide/ production. Coinhibition with CYP1A1/2 and CYP2E1 antibodies blocked CE formation by 38, 84, and 67 to 92% (n=3 individual samples) in mouse, rat, and human hepatic microsomes, respectively. Although CYP1A and 2E forms seem to be the most active catalysts of CE formation in liver, studies conducted with the mechanism-based inhibitor 5-phenyl-pentyne demonstrated that CYP2F2 is responsible for up to 67% of CE formation in whole mouse lung microsomes. In contrast to the CE pathway, coumarin 3-hydroxylation is a minor product of coumarin in liver microsomes from mice, rats, and humans and is catalyzed predominately by CYP3A and CYP1A forms, confirming that CE and 3-hydroxycoumarin are formed via distinct metabolic pathways.
PMID:11950775 Born SL et al; Drug Metab Dispos 30 (5): 483-7 (2002)
...To examine species differences in CYP2A function, liver microsomes from nine mammalian species (rat, mouse, hamster, rabbit, guinea pig, cat, dog, cynomolgus monkey and human were tested for their ability to catalyze the 7 alpha- and 15 alpha-hydroxylation of testosterone and the 7-hydroxylation of coumarin. Antibody against rat CYP2Al recognized one or more proteins in liver microsomes from all mammalian species examined. However, liver microsomes from cat, dog, cynomolgus monkey, and human catalyzed negligible rates of testosterone 7 alpha- and/or 15 alpha-hydroxylation, whereas rat and cat liver microsomes catalyzed negligible rates of coumarin 7-hydroxylation. Formation of 7-hydroxycoumarin accounted for a different proportion of the coumarin metabolites formed by liver microsomes from each of the various species examined. 7-Hydroxycoumarin was the major metabolite (>70%) in human and monkey, but only a minor metabolite (<1%) in rat. The 7-hydroxylation of coumarin by human liver microsomes was catalyzed by a single, high-affinity enzyme (Km 0.2-0.6 uM, which was markedly inhibited (>95%) by antibody against rat CYP2Al. The rate of coumarin 7-hydroxylation varied approximately 17-fold among liver microsomes from 22 human subjects. This variation was highly correlated (r2=0.956) with interindividual differences in the levels of CYP2A6... . These results indicate that CYP2A6 is largely or entirely responsible for catalyzing the 7-hydroxylation of coumarin in human liver, microsomes. Treatment of monkeys with phenobarbital or dexamethasone increased coumarin 7-hydroxylase activity, whereas treatment with beta-naphthoflavone caused a slight decr. In contrast to rats and mice, the expression of CYP2A enzymes in cynomolgus monkeys and humans was not sexually differentiated. Despite their structural similarity to coumarin, the anticoagulants dicumarol and warfarin do not appear to be substrates for CYP2A6. ...
PMID:1381906 Pearce R et al; Arch Biochem Biophys 298 (1): 211-25 (1992)
/The rat can/ hydroxylate coumarin in the 3-position. As can... the rabbit... .
LaDu, B.N., H.G. Mandel, and E.L. Way. Fundamentals of Drug Metabolism and Disposition. Baltimore: Williams and Wilkins, 1971., p. 191
The hepatic enzyme system, coumarin-7-hydroxylase, responsible for a high proportion of the hydroxylation of coumarin in cats, guinea pigs, hamsters, rabbits, and especially in man, is absent from the livers of ferrets, mice and rats. Rat liver contains an inhibitor of this enzyme.
The Chemical Society. Foreign Compound Metabolism in Mammals. Volume 1: A Review of the Literature Published Between 1960 and 1969. London: The Chemical Society, 1970., p. 399
For more Metabolism/Metabolites (Complete) data for COUMARIN (15 total), please visit the HSDB record page.
Coumarin has known human metabolites that include 3-Hydroxycoumarin, 7-Hydroxycoumarin, and Coumarin 3,4-epoxide.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Coumarin and some of its metabolites have been shown to inhibit glucose-6-phosphatase in liver and in liver microsomal preparation. It interferes with excision repair processes on ultra-violet-damaged DNA and with host cell reactivation of ultra-violet-irradiated phage T1 in E coli WP2.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V10 116
Both 4-hydroxycoumarin derivatives and indandiones (also known as oral anticoagulants) are antagonists of vitamin K. Their use as rodenticides is based on the inhibition of the vitamin K-dependent step in the synthesis of a number of blood coagulation factors. The vitamin K-dependent proteins ...in the coagulation cascade... are the procoagulant factors II (prothrombin), VII (proconvertin), IX (Christmas factor) and X (Stuart-Prower factor), and the coagulation-inhibiting proteins C and S. All these proteins are synthesized in the liver. Before they are released into the circulation the various precursor proteins undergo substantial (intracellular) post-translational modification. Vitamin K functions as a co-enzyme in one of these modifications, namely the carboxylation at well-defined positions of 10-12 glutamate residues into gamma-carboxyglutamate (Gla). The presence of these Gla residues is essential for the procoagulant activity of the various coagulations factors. Vitamin K hydroquinone (KH2) is the active co-enzyme, and its oxidation to vitamin K 2,3-epoxide (KO) provides the energy required for the carboxylation reaction. The epoxide is than recycled in two reduction steps mediated by the enzyme KO reductase... . The latter enzyme is the target enzyme for coumarin anticoagulants. Their blocking of the KO reductase leads to a rapid exhaustion of the supply of KH2, and thus to an effective prevention of the formation of Gla residues. This leads to an accumulation of non-carboxylated coagulation factor precursors in the liver. In some cases these precursors are processed further without being carboxylated, and (depending on the species) may appear in the circulation. At that stage the under-carboxylated proteins are designated as descarboxy coagulation factors. Normal coagulation factors circulate in the form of zymogens, which can only participate in the coagulation cascade after being activated by limited proteolytic degradation. Descarboxy coagulation factors have no procoagulant activity (i.e. they cannot be activated) and neither they can be converted into the active zymogens by vitamin K action. Whereas in anticoagulated humans high levels of circulating descarboxy coagulation factors are detectable, these levels are negligible in warfarin-treated rats and mice. /Anticoagulant rodenticides/
WHO; Environ Health Criteria 175: Anticoagulant Rodenticides p.46 (1995)


