



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
0
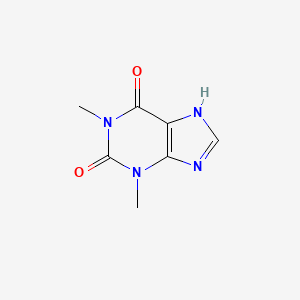

1. 1,3 Dimethylxanthine
2. 1,3-dimethylxanthine
3. 3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione
4. Accurbron
5. Aerobin
6. Aerolate
7. Afonilum Retard
8. Anhydrous, Theophylline
9. Aquaphyllin
10. Armophylline
11. Bronchoparat
12. Bronkodyl
13. Constant T
14. Constant-t
15. Constantt
16. Ct, Theo Von
17. Elixophyllin
18. Euphylong
19. Glycinate, Theophylline Sodium
20. Glycine Theophyllinate
21. Lodrane
22. Monospan
23. Nuelin
24. Nuelin S.a.
25. Quibron T Sr
26. Quibron T-sr
27. Quibron Tsr
28. Slo Phyllin
29. Slo-phyllin
30. Slophyllin
31. Sodium Glycinate, Theophylline
32. Somophyllin T
33. Somophyllin-t
34. Somophyllint
35. Sustaire
36. Synophylate
37. Theo 24
38. Theo Dur
39. Theo Von Ct
40. Theo-24
41. Theo-dur
42. Theo24
43. Theobid
44. Theocin
45. Theoconfin Continuous
46. Theodur
47. Theolair
48. Theolix
49. Theon
50. Theonite
51. Theopek
52. Theophyllinate, Glycine
53. Theophylline Anhydrous
54. Theophylline Sodium Glycinate
55. Theospan
56. Theostat
57. Theovent
58. Uniphyl
59. Uniphyllin
60. Uniphylline
61. Von Ct, Theo
1. 58-55-9
2. 1,3-dimethylxanthine
3. Elixophyllin
4. Theophyllin
5. Theophylline Anhydrous
6. Theolair
7. Nuelin
8. Respbid
9. Theocin
10. Theo-dur
11. Elixophylline
12. Pseudotheophylline
13. Lanophyllin
14. Theovent
15. Uniphyl
16. Slo-phyllin
17. Accurbron
18. Armophylline
19. Bronkodyl
20. Doraphyllin
21. Liquophylline
22. Maphylline
23. Medaphyllin
24. Optiphyllin
25. Parkophyllin
26. Synophylate
27. Teofyllamin
28. Theacitin
29. Theocontin
30. Uniphyllin
31. Xantivent
32. Aerolate
33. Elixicon
34. Solosin
35. Teolair
36. Theobid
37. Theofol
38. Theograd
39. Theolix
40. Elixex
41. Acet-theocin
42. Slo-bid
43. Theophyl-225
44. Aerolate Iii
45. Duraphyl
46. Euphylong
47. Sustaire
48. Austyn
49. Somophyllin-t
50. Constant-t
51. Teofilina
52. Asmax
53. Quibron T/sr
54. Theal Tablets
55. Theophylline, Anhydrous
56. 1,3-dimethyl-7h-purine-2,6-dione
57. Somophyllin-df
58. Bronkodyl Sr
59. Theoclair-sr
60. Theoclear La
61. Spophyllin Retard
62. Choledyl Sa
63. Quibron-t
64. Theoclear 80
65. Theophyline
66. Theo-11
67. Theostat 80
68. Theochron
69. 3,7-dihydro-1,3-dimethyl-1h-purine-2,6-dione
70. Synophylate-l.a. Cenules
71. Theo-dur-sprinkle
72. Aquaphyllin
73. Tefamin
74. Theo-24
75. Theodel
76. Unifyl
77. Theona P
78. 1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione
79. Labid
80. Euphylline
81. Elixomin
82. Theolixir
83. Theophyl
84. Bronkotabs
85. Elixophyllin Sr
86. Theophylline-sr
87. Somophyllin-crt
88. Aerolate Sr
89. Theo-organidin
90. Theophyl-sr
91. Gs 2591a
92. Uni-dur
93. Theoclear-80
94. Quibron-t/sr
95. T-phyl
96. Theoclear-200
97. 1h-purine-2,6-dione, 3,7-dihydro-1,3-dimethyl-
98. Xanthine, 1,3-dimethyl-
99. Nsc 2066
100. X 115
101. Quadrinal
102. Theobid Jr.
103. Theoclear L.a.-130
104. Theal Tabl.
105. 1,3-dimethyl-1h-purine-2,6(3h,7h)-dione
106. 1,3-dimethyl-2,3,6,7-tetrahydro-1h-purine-2,6-dione
107. Theophylline Melting Point Standard
108. Nsc-2066
109. Purine-2,6(1h,3h)-dione, 1,3-dimethyl-
110. 1,3-dimethyl-1h-purine-2,6(3h,9h)-dione
111. Aerobin
112. Theodur
113. Theopek
114. Theospan
115. Doxophylline Metabolite M3
116. Chembl190
117. Mls000069390
118. 1,3-dimethylxanthine;theo-24
119. Diffumal
120. Chebi:28177
121. Lasma
122. 0i55128jyk
123. Pro-vent
124. 58-55-9 (anhydrous)
125. Nsc-757346
126. 1246816-25-0
127. Teofilina [polish]
128. Ncgc00018117-07
129. Ncgc00018117-17
130. Mudrane
131. Pulmidur
132. Smr000058537
133. Teonova
134. Theokin
135. Hylate
136. Dsstox_cid_1336
137. Nuelin Sa
138. Bronchodid Duracap
139. Dsstox_rid_76090
140. Uniphyllin Continus
141. Dsstox_gsid_21336
142. 1h-purine-2,6-dione, 3,9-dihydro-1,3-dimethyl-
143. Teocen 200
144. Elixophyllin(e)
145. Cas-58-55-9
146. Theolair (tn)
147. Elixophyllin (tn)
148. Uniphyl (tn)
149. Quibron-t (tn)
150. Theodur G (tn)
151. Ccris 4729
152. Hsdb 3399
153. Sr-01000075195
154. Einecs 200-385-7
155. Theo-24 (tn)
156. Telbans
157. Physpa
158. Unii-0i55128jyk
159. Ai3-50216
160. Unicontin Cr
161. 4eoh
162. Aescin-iia
163. 1,3-dimethyl-2,6-dioxo-1,2,3,6-tetrahydropurine
164. Mfcd00079619
165. Theophylline Form Ii
166. Theophylline,anhydrous
167. Theophylline Solu-tion
168. Component Of Primatene
169. Xanthine,3-dimethyl-
170. Opera_id_76
171. Spectrum_001038
172. Theophylline (jp17)
173. 2a3a
174. Prestwick0_000820
175. Prestwick0_000873
176. Prestwick1_000820
177. Prestwick1_000873
178. Prestwick2_000820
179. Prestwick2_000873
180. Prestwick3_000820
181. Prestwick3_000873
182. Spectrum2_000842
183. Spectrum3_000672
184. Spectrum4_000353
185. Spectrum5_001232
186. Theophylline [mi]
187. 8-(2-furyl)theophylline
188. Upcmld-dp123
189. Component Of Theo-organidin
190. Ec 200-385-7
191. Schembl4915
192. Theophylline Anhydrous,(s)
193. 1,3-dimethyl-1,3,7-trihydropurine-2,6-dione
194. Theophylline Anhydrous, Usp
195. Lopac0_000014
196. Bspbio_000719
197. Bspbio_000945
198. Bspbio_002363
199. Component Of Dicurin Procaine
200. Gtpl413
201. Kbiogr_000785
202. Kbioss_001518
203. Mls002152943
204. Mls002153487
205. Mls004491910
206. Mls006011970
207. Bidd:er0557
208. Bidd:gt0151
209. Divk1c_000203
210. Spectrum1500568
211. Theophylline [usp-rs]
212. Theophylline [who-dd]
213. Spbio_000823
214. Spbio_002640
215. Spbio_002866
216. Bpbio1_000791
217. Bpbio1_001041
218. Schembl8312163
219. Dimenhydrinate Impurity A
220. Component Of Tedral (salt/mix)
221. Dtxsid5021336
222. Upcmld-dp123:001
223. Bcbcmap01_000071
224. Bdbm10847
225. Bdbm82053
226. Component Of Quibron (salt/mix)
227. Hms500k05
228. Kbio1_000203
229. Kbio2_001518
230. Kbio2_004086
231. Kbio2_006654
232. Kbio3_001583
233. Nsc2066
234. Component Of Hecadrol (salt/mix)
235. Ninds_000203
236. Hms1921e03
237. Hms2089a06
238. Hms2092m05
239. Hms2233e16
240. Hms3259o06
241. Hms3369n14
242. Pharmakon1600-01500568
243. Component Of Primatene (salt/mix)
244. Bcp30664
245. Component Of Bronkotabs (salt/mix)
246. Hy-b0809
247. Str01537
248. Theophylline 1.0 Mg/ml In Methanol
249. Theophylline, >=99.0% (hplc)
250. Theophylline-[1,3-15n2,13c]
251. Tox21_110827
252. Tox21_202375
253. Tox21_300028
254. Ccg-20301
255. Nsc757346
256. Pdsp1_001018
257. Pdsp1_001234
258. Pdsp2_001002
259. Pdsp2_001218
260. Stk397040
261. Theophylline (1,3-dimethylxanthine)
262. Theophylline Anhydrous [hsdb]
263. Zinc18043251
264. Theophylline,anhydrous [vandf]
265. Akos000120961
266. Akos005434016
267. Component Of Quibron Plus (salt/mix)
268. Tox21_110827_1
269. Component Of Quibron-t/sr (salt/mix)
270. Component Of Theo-organdin (salt/mix)
271. Component Of Theolair Plus (salt/mix)
272. Cs-4158
273. Db00277
274. Nc00542
275. Purine-2,3h)-dione, 1,3-dimethyl-
276. Sdccgmls-0066620.p001
277. Sdccgsbi-0050003.p005
278. Theophylline-[1,3-15n2,2-13c]
279. Component Of Theo-organidin (salt/mix)
280. Idi1_000203
281. Smp1_000291
282. Component Of Slo-phyllin Gg (salt/mix)
283. Ncgc00018117-01
284. Ncgc00018117-02
285. Ncgc00018117-03
286. Ncgc00018117-04
287. Ncgc00018117-05
288. Ncgc00018117-06
289. Ncgc00018117-08
290. Ncgc00018117-09
291. Ncgc00018117-10
292. Ncgc00018117-11
293. Ncgc00018117-12
294. Ncgc00018117-13
295. Ncgc00018117-14
296. Ncgc00018117-15
297. Ncgc00018117-16
298. Ncgc00018117-18
299. Ncgc00018117-19
300. Ncgc00018117-20
301. Ncgc00018117-23
302. Ncgc00018117-37
303. Ncgc00022112-03
304. Ncgc00022112-04
305. Ncgc00022112-05
306. Ncgc00022112-07
307. Ncgc00022112-08
308. Ncgc00022112-09
309. Ncgc00022112-10
310. Ncgc00022112-11
311. Ncgc00022112-12
312. Ncgc00254040-01
313. Ncgc00259924-01
314. Wln: T56 Bm Dn Fnvnvj F1 H1
315. Ac-20328
316. Component Of Dicurin Procaine (salt/mix)
317. Nci60_001736
318. Smr003435989
319. Theophylline, Anhydrous, >=99%, Powder
320. Component Of Primatene Tablets (salt/mix)
321. Purine,6(1h,3h)-dione, 1,3-dimethyl-
322. Sbi-0050003.p004
323. Component Of Mudrane Gg Elixir (salt/mix)
324. Db-053224
325. Theophylline 100 Microg/ml In Acetonitrile
326. 1h-purine-2, 3,7-dihydro-1,3-dimethyl-
327. Ab00052106
328. Ft-0631259
329. Ft-0675140
330. N1442
331. T0179
332. C07130
333. D00371
334. D85002
335. Dimenhydrinate Impurity A [ep Impurity]
336. Pentoxifylline Impurity C [ep Impurity]
337. Ab00052106-20
338. Ab00052106-22
339. Ab00052106-23
340. Ab00052106_24
341. Ab00052106_25
342. Ab00052106_26
343. 1,3-dimethyl-3,9-dihydro-1h-purine-2,6-dione
344. A900445
345. L000595
346. Q407308
347. 1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione #
348. Q-201819
349. Sr-01000075195-1
350. Sr-01000075195-3
351. Sr-01000075195-5
352. 1,3-dimethyl-6-hydroxy-1,3-dihydro-2h-purin-2-one
353. Brd-k97799481-001-02-0
354. Brd-k97799481-001-12-9
355. Brd-k97799481-002-03-6
356. 1h-purine-2,6-dione, 3,7-dihydro-1,3-dimethyl-, Mo
357. F2173-0145
358. Z271004650
359. A3133d49-aab6-49a1-b60b-b5198f327d3f
360. Theophylline, British Pharmacopoeia (bp) Reference Standard
361. Theophylline, European Pharmacopoeia (ep) Reference Standard
362. (8alpha, 9s)-10,11-dihydro-6'-methoxycinchonan-9-amine Trihydrochloride, Min. 90%
363. Theophylline Melting Point Standard, United States Pharmacopeia (usp) Reference Standard
364. Theophylline Solution, 1.0 Mg/ml In Methanol, Ampule Of 1 Ml, Certified Reference Material
365. Theophylline, Pharmaceutical Secondary Standard; Certified Reference Material
366. 50857-74-4
367. 75448-53-2
368. Theo-24;1,3-dimethyl-3,7-dihydro-1h-purine-2,6-dione ;1,3-dimethylxanthine;1,3-dimethyl-xanthin
369. Theophylline Melting Point Standard, Pharmaceutical Secondary Standard; Certified Reference Material
370. Theophylline, Certified Reference Material, United States Pharmacopeia (usp) Reference Standard
| Molecular Weight | 180.16 g/mol |
|---|---|
| Molecular Formula | C7H8N4O2 |
| XLogP3 | 0 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 180.06472551 g/mol |
| Monoisotopic Mass | 180.06472551 g/mol |
| Topological Polar Surface Area | 69.3 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 267 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 14 | |
|---|---|
| Drug Name | Choledyl sa |
| PubMed Health | Theophylline |
| Drug Classes | Bronchodilator, Methylxanthine |
| Drug Label | Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with a bitter taste. Anhydrous theophylline has the chemical name 1H-Purine- 2,6-dione, 3,7-dihydro-1,3 -dimethyl-, and is represented by... |
| Active Ingredient | Oxtriphylline |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 600mg; 400mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 2 of 14 | |
|---|---|
| Drug Name | Elixophyllin |
| PubMed Health | Theophylline |
| Drug Classes | Bronchodilator, Methylxanthine |
| Active Ingredient | Theophylline |
| Dosage Form | Solution, elixir |
| Route | Oral |
| Strength | 80mg/15ml |
| Market Status | Prescription |
| Company | Sun Pharm Inds |
| 3 of 14 | |
|---|---|
| Drug Name | Theo-24 |
| PubMed Health | Theophylline |
| Drug Classes | Bronchodilator, Methylxanthine |
| Drug Label | Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with a bitter taste. Anhydrous theophylline has the chemical name 1H-Purine-2,6-dione, 3,7-dihydro-1,3-dimethyl-, and is represented by th... |
| Active Ingredient | Theophylline |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 200mg; 400mg; 300mg; 100mg |
| Market Status | Prescription |
| Company | Actient Pharms |
| 4 of 14 | |
|---|---|
| Drug Name | Theochron |
| Drug Label | Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with a bitter taste. Anhydrous theophylline has the chemical name 1H-Purine-2,6-dione,3,7-dihydro-1,3-dimethyl-, and is represented by the... |
| Active Ingredient | Theophylline |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 200mg; 100mg |
| Market Status | Prescription |
| Company | Sun Pharm Inds |
| 5 of 14 | |
|---|---|
| Drug Name | Theolair |
| Drug Label | Uniphyl (theophylline, anhydrous) Tablets in a controlled-release system allows a 24-hour dosing interval for appropriate patients.Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with... |
| Active Ingredient | Theophylline |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 125mg |
| Market Status | Prescription |
| Company | Medicis |
| 6 of 14 | |
|---|---|
| Drug Name | Theophylline |
| Active Ingredient | Theophylline |
| Dosage Form | Tablet, extended release; Solution |
| Route | oral; Oral |
| Strength | 200mg; 80mg/15ml; 600mg; 400mg; 300mg; 100mg; 450mg |
| Market Status | Prescription |
| Company | Silarx; Alembic; Nostrum; Rhodes Pharms; Glenmark Generics; Teva Pharms; Pliva; Tris Pharma |
| 7 of 14 | |
|---|---|
| Drug Name | Uniphyl |
| Active Ingredient | Theophylline |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 400mg |
| Market Status | Prescription |
| Company | Rhodes Pharms |
| 8 of 14 | |
|---|---|
| Drug Name | Choledyl sa |
| PubMed Health | Theophylline |
| Drug Classes | Bronchodilator, Methylxanthine |
| Drug Label | Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with a bitter taste. Anhydrous theophylline has the chemical name 1H-Purine- 2,6-dione, 3,7-dihydro-1,3 -dimethyl-, and is represented by... |
| Active Ingredient | Oxtriphylline |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 600mg; 400mg |
| Market Status | Prescription |
| Company | Warner Chilcott |
| 9 of 14 | |
|---|---|
| Drug Name | Elixophyllin |
| PubMed Health | Theophylline |
| Drug Classes | Bronchodilator, Methylxanthine |
| Active Ingredient | Theophylline |
| Dosage Form | Solution, elixir |
| Route | Oral |
| Strength | 80mg/15ml |
| Market Status | Prescription |
| Company | Sun Pharm Inds |
| 10 of 14 | |
|---|---|
| Drug Name | Theo-24 |
| PubMed Health | Theophylline |
| Drug Classes | Bronchodilator, Methylxanthine |
| Drug Label | Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with a bitter taste. Anhydrous theophylline has the chemical name 1H-Purine-2,6-dione, 3,7-dihydro-1,3-dimethyl-, and is represented by th... |
| Active Ingredient | Theophylline |
| Dosage Form | Capsule, extended release |
| Route | Oral |
| Strength | 200mg; 400mg; 300mg; 100mg |
| Market Status | Prescription |
| Company | Actient Pharms |
| 11 of 14 | |
|---|---|
| Drug Name | Theochron |
| Drug Label | Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with a bitter taste. Anhydrous theophylline has the chemical name 1H-Purine-2,6-dione,3,7-dihydro-1,3-dimethyl-, and is represented by the... |
| Active Ingredient | Theophylline |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 200mg; 100mg |
| Market Status | Prescription |
| Company | Sun Pharm Inds |
| 12 of 14 | |
|---|---|
| Drug Name | Theolair |
| Drug Label | Uniphyl (theophylline, anhydrous) Tablets in a controlled-release system allows a 24-hour dosing interval for appropriate patients.Theophylline is structurally classified as a methylxanthine. It occurs as a white, odorless, crystalline powder with... |
| Active Ingredient | Theophylline |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 250mg; 125mg |
| Market Status | Prescription |
| Company | Medicis |
| 13 of 14 | |
|---|---|
| Drug Name | Theophylline |
| Active Ingredient | Theophylline |
| Dosage Form | Tablet, extended release; Solution |
| Route | oral; Oral |
| Strength | 200mg; 80mg/15ml; 600mg; 400mg; 300mg; 100mg; 450mg |
| Market Status | Prescription |
| Company | Silarx; Alembic; Nostrum; Rhodes Pharms; Glenmark Generics; Teva Pharms; Pliva; Tris Pharma |
| 14 of 14 | |
|---|---|
| Drug Name | Uniphyl |
| Active Ingredient | Theophylline |
| Dosage Form | Tablet, extended release |
| Route | Oral |
| Strength | 400mg |
| Market Status | Prescription |
| Company | Rhodes Pharms |
Bronchodilator Agents; Phosphodiesterase Inhibitors; Purinergic P1 Receptor Antagonists; Vasodilator Agents
National Library of Medicine's Medical Subject Headings. Theophylline. Online file (MeSH, 2016). Available from, as of June 24, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Theophylline is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 6, 2016: https://clinicaltrials.gov/search/intervention=theophylline
Limited evidence suggests that an IV xanthine derivative (e.g., theophylline, aminophylline) could be beneficial as add-on therapy in children who are admitted to an intensive care unit (ICU) for severe exacerbations of asthma not controlled by inhaled and IV beta2-adrenergic agonists, ipratropium bromide, and IV corticosteroids; however, the efficacy of such add-on IV theophylline therapy has not been established in adults.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3602
Theophylline is indicated for the treatment of the symptoms and reversible airflow obstruction associated with chronic asthma and other chronic lung diseases, e.g., emphysema and chronic bronchitis. /Included in US product labeling/
NIH; DailyMed. Current Medication Information for Theophylline (Anhydrous) Tablet, Extended Release (Updated: November 2014). Available from, as of July 21, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=038c2b07-8028-4dc4-847c-adafe1b0e81a
For more Therapeutic Uses (Complete) data for THEOPHYLLINE (10 total), please visit the HSDB record page.
When administered rectally as suppositories (dosage form no longer commercially available in the US), theophyllines have caused rectal irritation and inflammation. /Theophyllines/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3605
Theophyllines may also produce transiently increased urinary frequency, dehydration, twitching of fingers and hands, tachypnea, and elevated serum AST (SGOT) concentrations. Hypersensitivity reactions characterized by urticaria, generalized pruritus, and angioedema have been reported with aminophylline administration. A contact-type dermatitis, caused by hypersensitivity to the ethylenediamine component of aminophylline, has also been reported. Bone marrow suppression, leukopenia, thrombocytopenia, and hemorrhagic diathesis have also been reported, but their association with theophylline therapy is questionable. Other adverse effects of theophyllines include albuminuria, increased urinary excretion of renal tubular cells and erythrocytes, hyperglycemia, and syndrome of inappropriate secretion of antidiuretic hormone (SIADH). /Theophyllines/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3605
Adverse cardiovascular effects of theophyllines include palpitation, sinus tachycardia, extrasystoles, and increased pulse rate. These adverse cardiovascular effects are usually mild and transient. Flushing, hypotension, circulatory failure, and ventricular arrhythmias may also occur. /Theophyllines/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3605
Theophyllines produce GI irritation and CNS stimulation following administration by any route. Theophyllines are all somewhat irritating to gastric mucosa; the importance of reported differences among the individual agents is doubtful. The most common adverse GI effects (both locally and centrally mediated) include nausea, vomiting, epigastric pain, abdominal cramps, anorexia, and, rarely, diarrhea. Hematemesis has also occurred. Adverse CNS effects, which are often more severe in children than in adults, include headache, irritability, restlessness, nervousness, insomnia, dizziness, reflex hyperexcitability, and seizures. Reduction of theophylline dosage usually reduces the incidence and severity of adverse gastric and CNS effects; however, if these adverse effects persist, the drug may have to be withdrawn. The drugs may be administered orally before or after meals, with a full glass of liquid, or with antacids to minimize locally mediated GI irritation. /Theophyllines/
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3605
For more Drug Warnings (Complete) data for THEOPHYLLINE (19 total), please visit the HSDB record page.
For the treatment of the symptoms and reversible airflow obstruction associated with chronic asthma and other chronic lung diseases, such as emphysema and chronic bronchitis.
FDA Label
Theophylline, an xanthine derivative chemically similar to caffeine and theobromine, is used to treat asthma and bronchospasm. Theophylline has two distinct actions in the airways of patients with reversible (asthmatic) obstruction; smooth muscle relaxation (i.e., bronchodilation) and suppression of the response of the airways to stimuli (i.e., non-bronchodilator prophylactic effects).
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)
Bronchodilator Agents
Agents that cause an increase in the expansion of a bronchus or bronchial tubes. (See all compounds classified as Bronchodilator Agents.)
Phosphodiesterase Inhibitors
Compounds which inhibit or antagonize the biosynthesis or actions of phosphodiesterases. (See all compounds classified as Phosphodiesterase Inhibitors.)
Purinergic P1 Receptor Antagonists
Compounds that bind to and block the stimulation of PURINERGIC P1 RECEPTORS. (See all compounds classified as Purinergic P1 Receptor Antagonists.)
R03DA04
S76 | LUXPHARMA | Pharmaceuticals Marketed in Luxembourg | Pharmaceuticals marketed in Luxembourg, as published by d'Gesondheetskeess (CNS, la caisse nationale de sante, www.cns.lu), mapped by name to structures using CompTox by R. Singh et al. (in prep.). List downloaded from https://cns.public.lu/en/legislations/textes-coordonnes/liste-med-comm.html. Dataset DOI:10.5281/zenodo.4587355
R - Respiratory system
R03 - Drugs for obstructive airway diseases
R03D - Other systemic drugs for obstructive airway diseases
R03DA - Xanthines
R03DA04 - Theophylline
Absorption
Theophylline is rapidly and completely absorbed after oral administration in solution or immediate-release solid oral dosage form.
Route of Elimination
Theophylline does not undergo any appreciable pre-systemic elimination, distributes freely into fat-free tissues and is extensively metabolized in the liver. Renal excretion of unchanged theophylline in neonates amounts to about 50% of the dose, compared to about 10% in children older than three months and in adults.
Volume of Distribution
0.3 to 0.7 L/kg
Clearance
0.29 mL/kg/min [Premature neonates, postnatal age 3-15 days]
0.64 mL/kg/min [Premature neonates, postnatal age 25-57 days]
1.7 mL/kg/min [Children 1-4 years]
1.6 mL/kg/min [Children 4-12 years]
0.9 mL/kg/min [Children 13-15 years]
1.4 mL/kg/min [Children 16-17 years]
0.65 mL/kg/min [Adults (16-60 years), otherwise healthy non-smoking asthmatics]
0.41 mL/kg/min [Elderly (>60 years), non-smokers with normal cardiac, liver, and renal function]
0.33 mL/kg/min [Acute pulmonary edema]
0.54 mL/kg/min [COPD >60 years, stable, non-smoker >1 year]
0.48 mL/kg/min [COPD with cor pulmonale]
1.25 mL/kg/min [Cystic fibrosis (14-28 years)]
0.31 mL/kg/min [Liver disease cirrhosis]
0.35 mL/kg/min [acute hepatitis]
0.65 mL/kg/min [cholestasis]
0.47 mL/kg/min [Sepsis with multi-organ failure]
0.38 mL/kg/min [hypothyroid]
0.8 mL/kg/min [hyperthyroid]
When administered IM, theophylline is usually absorbed slowly and incompletely. Rectal suppositories (no longer commercially available in the US) are slowly and erratically absorbed, regardless of whether the suppository base is hydrophilic or lipophilic.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3607
In neonates, approximately 50% of the theophylline dose is excreted unchanged in the urine. Beyond the first three months of life, approximately 10% of the theophylline dose is excreted unchanged in the urine.
NIH; DailyMed. Current Medication Information for Theophylline (Anhydrous) Tablet, Extended Release (Updated: November 2014). Available from, as of July 21, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=038c2b07-8028-4dc4-847c-adafe1b0e81a
Dissolution appears to be the rate-limiting step in the absorption of oral theophylline. Under the acidic conditions of the stomach, the theophylline salts and compounds release free theophylline. ... Microcrystalline dosage forms and oral solutions of theophyllines are absorbed more rapidly, but not to a greater extent, than are uncoated tablets. Although the rate of absorption is slower, extended-release preparations (capsules and tablets) of theophylline are generally absorbed to the same extent as uncoated tablets; however, the actual rate of absorption of extended-release preparations may differ. Extended-release preparations of theophyllines have been formulated to release the drug at various rates suitable for dosing every 8-12, 12, or 24 hours; however, the actual dosing frequency for a given patient depends on their individual pharmacokinetic parameters. Since the rate and extent of absorption may differ between various extended-release preparations and sometimes between different dosage sizes of the same preparation, patients should generally be stabilized on a given preparation; substitution of one extended-release preparation for another should generally only be made when the preparations have been shown to be equivalent and/or the patient is evaluated pharmacokinetically during the transition period. Absorption of theophyllines may also be delayed, but is generally not reduced, by the presence of food in the GI tract; however, the effect of food on the absorption of extended-release preparations appears to be variable, and the manufacturer's recommendations for administration of specific preparations should be followed.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3607
IV theophylline produces the highest and most rapid serum theophylline concentration. ... Slightly lower or equal peak serum concentrations are reached after oral administration of equal amounts of theophylline in uncoated tablet, capsule, or liquid formulations. Following oral administration of theophylline capsules or uncoated tablets, peak serum concentrations are usually reached in 1-2 hours. Peak serum theophylline concentrations are usually obtained after about 1 hour when theophylline oral solutions or microcrystalline tablets are administered. Enteric-coated theophylline tablets produce variable serum concentrations which usually peak at about 5 hours. Single doses of extended-release theophylline capsules or tablets usually produce peak serum concentrations after 4 hours, but commercial products vary in their rates and completeness of absorption. Extended-release theophylline preparations are generally associated with relatively small fluctuations in steady-state peak and trough serum concentration; however, clinically important steady-state peak-trough differences may occur in individuals who rapidly eliminate theophylline. Theophylline retention enemas usually produce peak serum concentrations in 1-2 hours. Serum theophylline concentrations generally have been apparent 3-5 hours after administration of the drug as rectal suppositories (no longer commercially available in the US).
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3607
For more Absorption, Distribution and Excretion (Complete) data for THEOPHYLLINE (19 total), please visit the HSDB record page.
Hepatic. Biotransformation takes place through demethylation to 1-methylxanthine and 3-methylxanthine and hydroxylation to 1,3-dimethyluric acid. 1-methylxanthine is further hydroxylated, by xanthine oxidase, to 1-methyluric acid. About 6% of a theophylline dose is N-methylated to caffeine. Caffeine and 3-methylxanthine are the only theophylline metabolites with pharmacologic activity.
Both the N-demethylation and hydroxylation pathways of theophylline biotransformation are capacity-limited. Due to the wide intersubject variability of the rate of theophylline metabolism, non-linearity of elimination may begin in some patients at serum theophylline concentrations <10 ug/mL. Since this non-linearity results in more than proportional changes in serum theophylline concentrations with changes in dose, it is advisable to make increases or decreases in dose in small increments in order to achieve desired changes in serum theophylline concentrations. Accurate prediction of dose-dependency of theophylline metabolism in patients a priori is not possible, but patients with very high initial clearance rates (i.e., low steady-state serum theophylline concentrations at above average doses) have the greatest likelihood of experiencing large changes in serum theophylline concentration in response to dosage changes.
NIH; DailyMed. Current Medication Information for Theophylline (Anhydrous) Tablet, Extended Release (Updated: November 2014). Available from, as of July 21, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=038c2b07-8028-4dc4-847c-adafe1b0e81a
Caffeine and 3-methylxanthine are the only theophylline metabolites with pharmacologic activity. 3-methylxanthine has approximately one tenth the pharmacologic activity of theophylline and serum concentrations in adults with normal renal function are <1 ug/mL. In patients with end-stage renal disease, 3-methylxanthine may accumulate to concentrations that approximate the unmetabolized theophylline concentration. Caffeine concentrations are usually undetectable in adults regardless of renal function. In neonates, caffeine may accumulate to concentrations that approximate the unmetabolized theophylline concentration and thus, exert a pharmacologic effect.
NIH; DailyMed. Current Medication Information for Theophylline (Anhydrous) Tablet, Extended Release (Updated: November 2014). Available from, as of July 21, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=038c2b07-8028-4dc4-847c-adafe1b0e81a
Theophylline is metabolized by the liver to 1,3-dimethyluric acid, 1-methyluric acid, and 3-methylxanthine. ... Individuals metabolize theophylline at different rates; however, individual metabolism of the drug is generally reproducible. Theophylline and its metabolites are excreted mainly by the kidneys. Renal clearance of the drug, however, contributes only 8-12% of the overall plasma clearance of theophylline. Small amounts of theophylline are excreted in feces unchanged.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3607
Unchanged theophylline (35% of urinary radioactivity) and 1,3-dimethyluric acid (34%) are the main compounds excreted in urine, followed by 1-methyluric acid (18%), 3-methylxanthine (3%) and unidentified polar metabolites (4.8%). Theophylline metabolism was impaired in rats at day 18 of gestation, as shown by increased excretion of theophylline (73%); this was explained by a decreased formation of 1,3-dimethyluric acid (-68%) and 1-methyluric acid (-30%). Essentially similar results were obtained with pregnant baboons. Each animal species is characterized by differences in the profile of the metabolites recovered in urine; in addition, quantitative differences in theophylline metabolic pathways were seen even in different strains of mice.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 397 (1991)
For more Metabolism/Metabolites (Complete) data for THEOPHYLLINE (7 total), please visit the HSDB record page.
Theophylline has known human metabolites that include 1,3-Dimethyluric acid, 1-Methylxanthine, and 3-Methylxanthine.
Theophylline is a known human metabolite of caffeine.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
8 hours
In maintenance-dose theophylline schedules, serum concentrations among patients vary at least sixfold and serum half-lives exhibit wide interpatient variation because of differences in rate of metabolism. Serum half lives ranges from about 3-12.8 (average 7-9) hours in otherwise healthy, nonsmoking asthmatic adults, from about 1.5-9.5 hours in children, and from about 15-58 hours in premature infants. ... When compared with that of otherwise healthy, nonsmoking asthmatic adults, the serum half life of theophylline may be increased and total body clearance decreased in patients with congestive heart failure, chronic obstructive pulmonary disease, cor pulmonale, or liver disease, and in geriatric patients. In cigarette and/or marijuana smokers, theophylline serum half life averages 4-5 hours and total body clearance is increased compared with nonsmokers.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016, p. 3607
Theophylline is distributed rapidly within the body, and plasma half-times were 5.7-11.5 hr in dogs, 11 hr in pigs, 7.8 hr in cats, 3.8-5.5 hr in rabbits and 1.2-4 hr in rats. At higher doses, rats had longer half-times probably because of a combination of increased diuresis and saturation of the metabolism.
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V51 397 (1991)
Theophylline relaxes the smooth muscle of the bronchial airways and pulmonary blood vessels and reduces airway responsiveness to histamine, methacholine, adenosine, and allergen. Theophylline competitively inhibits type III and type IV phosphodiesterase (PDE), the enzyme responsible for breaking down cyclic AMP in smooth muscle cells, possibly resulting in bronchodilation. Theophylline also binds to the adenosine A2B receptor and blocks adenosine mediated bronchoconstriction. In inflammatory states, theophylline activates histone deacetylase to prevent transcription of inflammatory genes that require the acetylation of histones for transcription to begin.
Theophylline is an old drug experiencing a renaissance owing to its beneficial antiinflammatory effects in chronic respiratory diseases, such as asthma and chronic obstructive pulmonary disease. Multiple modes of antiinflammatory action have been reported, including inhibition of the enzymes that degrade cAMP-phosphodiesterase (PDE). Using primary cultures of airway smooth muscle (ASM) cells, we recently revealed that PDE4 inhibitors can potentiate the antiinflammatory action of beta2-agonists by augmenting cAMP-dependent expression of the phosphatase that deactivates mitogen-activated protein kinase (MAPK)-MAPK phosphatase (MKP)-1. Therefore, the aim of this study was to address whether theophylline repressed cytokine production in a similar, PDE-dependent, MKP-1-mediated manner. Notably, theophylline did not potentiate cAMP release from ASM cells treated with the long-acting beta2-agonist formoterol. Moreover, theophylline (0.1-10 uM) did not increase formoterol-induced MKP-1 messenger RNA expression nor protein up-regulation, consistent with the lack of cAMP generation. However, theophylline (at 10 uM) was antiinflammatory and repressed secretion of the neutrophil chemoattractant cytokine IL-8, which is produced in response to TNF-a. Because theophylline's effects were independent of PDE4 inhibition or antiinflammatory MKP-1, we then wished to elucidate the novel mechanisms responsible. We investigated the impact of theophylline on protein phosphatase (PP) 2A, a master controller of multiple inflammatory signaling pathways, and show that theophylline increases TNF-a-induced PP2A activity in ASM cells. Confirmatory results were obtained in A549 lung epithelial cells. PP2A activators have beneficial effects in ex vivo and in vivo models of respiratory disease. Thus, our study is the first to link theophylline with PP2A activation as a novel mechanism to control respiratory inflammation.
PMID:26574643 Patel BS et al; Am J Respir Cell Mol Biol 54 (6): 792-801 (2016)
Theophylline has two distinct actions in the airways of patients with reversible obstruction; smooth muscle relaxation (i.e., bronchodilation) and suppression of the response of the airways to stimuli (i.e., non-bronchodilator prophylactic effects). While the mechanisms of action of theophylline are not known with certainty, studies in animals suggest that bronchodilatation is mediated by the inhibition of two isozymes of phosphodiesterase (PDE III and, to a lesser extent, PDE IV) while non-bronchodilator prophylactic actions are probably mediated through one or more different molecular mechanisms, that do not involve inhibition of PDE III or antagonism of adenosine receptors. Some of the adverse effects associated with theophylline appear to be mediated by inhibition of PDE III (e.g., hypotension, tachycardia, headache, and emesis) and adenosine receptor antagonism (e.g., alterations in cerebral blood flow). Theophylline increases the force of contraction of diaphragmatic muscles. This action appears to be due to enhancement of calcium uptake through an adenosine-mediated channel.
NIH; DailyMed. Current Medication Information for Theophylline (Anhydrous) Tablet, Extended Release (Updated: November 2014). Available from, as of July 21, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=038c2b07-8028-4dc4-847c-adafe1b0e81a
Lung deflation and inflation during cardiac surgery with cardiopulmonary bypass contributes to pulmonary dysfunction postoperatively. Theophylline treatment for lung diseases has traditionally been thought to act by phosphodiesterase inhibition; however, increasing evidence has suggested other plausible mechanisms. We investigated the effects of deflation and reinflation on signaling pathways (p38-mitogen-activated protein kinase [MAPK], extracellular signal-regulated kinase 1 and 2 [ERK1/2], and Akt) and whether theophylline influences the deflation-induced lung injury and associated signaling. Isolated rat lungs were perfused (15 mL/min) with deoxygenated rat blood in bicarbonate buffer and ventilated. After 20 minutes' equilibration, the lungs were deflated (60 minutes, aerobic perfusion 1.5 mL/min), followed by reinflation (60 minutes, anaerobic reperfusion 15 mL/min). Compliance, vascular resistance, and kinase phosphorylation were assessed during deflation and reinflation. The effects of SB203580 (50 uM), a p38-MAPK inhibitor, and theophylline (0.083 mM [therapeutic] or 3 mM [supratherapeutic]) on physiology and signaling were studied. Deflation reduced compliance by 44% compared with continuously ventilated lungs. p38-MAPK and Akt phosphorylation increased (three to fivefold) during deflation and reinflation, and ERK1/2 phosphorylation increased (approximately twofold) during reinflation. SB203580 had no effect on lung physiology or ERK1/2 and Akt activation. Both theophylline doses increased cyclic adenosine monophosphate, but only 3 mM theophylline improved compliance. p38-MAPK phosphorylation was not affected by theophylline; 0.083 mM theophylline inhibited reinflation-induced ERK1/2 phosphorylation (72% + or - 3%); and 3 mM theophylline inhibited Akt phosphorylation during deflation (75% + or - 5%) and reinflation (87% + or - 4%). Lung deflation and reinflation stimulates differential p38-MAPK, ERK1/2, and Akt activation, suggesting a role in lung injury during cardiopulmonary bypass. However, p38-MAPK was not involved in the compromised compliance. A supratherapeutic theophylline dose protected lungs against deflation-induced injury and was associated with inhibition of phosphoinositide 3-kinase/Akt rather than phosphodiesterase.
PMID:24841445 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4226635 Markou T, Chambers DJ; J Thorac Cardiovasc Surg 148 (5): 2335-44 (2014)
Theophylline and aminophylline have been widely used as inhibitors of phosphodiesterase when examining the role of cAMP in regulating cell function. In reality, however, these phosphodiesterase inhibitors may have additional sites of action that could complicate the interpretation of the results. These additional sites of action could include antagonism of inhibitory adenosine autoreceptors and release of intracellular calcium. The purpose of the present study was to determine which of the above three is the primary mechanism by which theophylline and aminophylline affect transmitter release at the mammalian neuromuscular junction. Quantal release measurements were made using intracellular recording techniques. A variety of drugs were used to elucidate this pathway. Isoproterenol, an adenylate cyclase activator, was first used to establish the effect of enhanced levels of cAMP. Theophylline application on its own or in the presence of a drug combination that blocked the adenosine receptor and phosphodiesterase pathways caused significant release depression, opposite to what is expected if it was functioning to enhance cAMP levels. However, when applied in the presence of a drug combination that blocked the adenosine receptor, phosphodiesterase and intracellular ryanodine calcium pathways, theophylline was unable to depress release. Therefore, it was concluded that the major mechanism of action of theophylline is depression of transmitter release by causing the release of intracellular calcium. Aminophylline application alone resulted in a significant enhancement of release. However, when coupled with an adenosine receptor blocker, the ability of aminophylline to enhance transmitter release was blocked, suggesting that its dominant mechanism of action is adenosine receptor inhibition. Taken together, these results indicate that the use of theophylline and aminophylline is inappropriate when examining the role of cAMP at the mammalian neuromuscular junction.
PMID:16700879 Nickels TJ et al; Clin Exp Pharmacol Physiol 33 (5-6): 465-70 (2006)








