



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
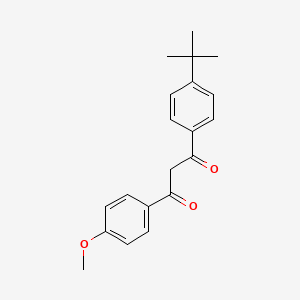

1. 4-tert-butyl-4'-methoxydibenzoylmethane
2. Bmdbm Cpd
3. Butyl-methoxydibenzoylmethane
4. Parsol 1789
5. Parsol-1789
1. 70356-09-1
2. Parsol 1789
3. Butyl Methoxydibenzoylmethane
4. 1-(4-tert-butylphenyl)-3-(4-methoxyphenyl)propane-1,3-dione
5. Escalol 517
6. Eusolex 9020
7. 4-tert-butyl-4'-methoxy-dibenzoylmethane
8. Neoheliopan 357
9. 1-(4-tert-butylphenyl)-3-(4-methoxyphenyl)-1,3-propanedione
10. 1-(4-(tert-butyl)phenyl)-3-(4-methoxyphenyl)propane-1,3-dione
11. 1,3-propanedione, 1-[4-(1,1-dimethylethyl)phenyl]-3-(4-methoxyphenyl)-
12. G63qqf2nox
13. Nsc-758680
14. 1-(p-tert-butylphenyl)-3-(p-methoxyphenyl)-1,3-propanedione
15. 1-(4-(1,1-dimethylethyl)phenyl)-3-(4-methoxyphenyl)-1,3-propanedione
16. Ncgc00095112-01
17. 1,3-propanedione, 1-(4-(1,1-dimethylethyl)phenyl)-3-(4-methoxyphenyl)-
18. Dsstox_cid_24829
19. Dsstox_rid_80510
20. Dsstox_gsid_44829
21. 87075-14-7
22. Avobenzona
23. Avobenzonum
24. Avobenzonum [inn-latin]
25. 1-[4-(1,1-dimethylethyl)phenyl]-3-(4-methoxyphenyl)-1,3-propanedione
26. Avobenzona [inn-spanish]
27. Parsol A
28. Smr001562107
29. Cas-70356-09-1
30. Hsdb 7423
31. Einecs 274-581-6
32. Unii-g63qqf2nox
33. 4-tert-butyl-4'-methoxydibenzoylmethane
34. Avobenzone [usan:usp:inn]
35. 4-methoxy-4'-tert-butyldibenzoylmethane
36. Spectrum_001715
37. Bf2avb
38. Avobenzone [mi]
39. Specplus_000764
40. Avobenzone (usp/inn)
41. Avobenzone [inn]
42. Spectrum2_001663
43. Spectrum3_000990
44. Spectrum4_001116
45. Spectrum5_001358
46. Avobenzone [hsdb]
47. Avobenzone [usan]
48. Avobenzone(parsol 1789)
49. Ec 274-581-6
50. Avobenzone [mart.]
51. Avobenzone [usp-rs]
52. Avobenzone [who-dd]
53. Avobenzone (parsol 1789)
54. Schembl15650
55. Bspbio_002659
56. Kbiogr_001592
57. Kbioss_002195
58. Zinc973
59. Mls002695918
60. Mls006010050
61. Bidd:er0196
62. Divk1c_006860
63. Spectrum1504190
64. Spbio_001845
65. Avobenzone, Analytical Standard
66. Avobenzone [orange Book]
67. Chembl1200522
68. Dtxsid9044829
69. Kbio1_001804
70. Kbio2_002195
71. Kbio2_004763
72. Kbio2_007331
73. Kbio3_001879
74. Avobenzone [usp Impurity]
75. Chebi:134751
76. Avobenzone [usp Monograph]
77. Hms1922f17
78. Hms2093c04
79. Hms3655c22
80. Hms3715f14
81. Pharmakon1600-01504190
82. Hy-b0316
83. Tox21_111427
84. Tox21_202796
85. Ccg-39080
86. Mfcd00210252
87. Nsc758680
88. S1904
89. 1-(4-(1,1-dimethylethyl)phenyl)-3-(4-methoxyphenyl)-1,3-propanedi- One
90. 1-(4-(1,1-dimethylethyl)phenyl)-3-(4-methoxyphenyl)propane-1,3-dione
91. Akos015838120
92. Tox21_111427_1
93. Ac-1682
94. Db09495
95. Nsc 758680
96. Anthelios Sx Component Avobenzone
97. Ncgc00095112-02
98. Ncgc00095112-03
99. Ncgc00095112-04
100. Ncgc00095112-05
101. Ncgc00260342-01
102. Rac-erythro Methylphenidate Hydrochloride
103. 23644-60-2
104. As-12797
105. Capital Soleil Component Avobenzone
106. Shade Uvaguard Component Avobenzone
107. Parsol 1789 100 Microg/ml In Methanol
108. Sbi-0052777.p002
109. Avobenzone Component Of Anthelios Sx
110. Butyl Methoxydibenzoylmethane [inci]
111. Avobenzone Component Of Capital Soleil
112. Avobenzone Component Of Shade Uvaguard
113. B3382
114. Ft-0623334
115. Sw219665-1
116. Parsol 1789 100 Microg/ml In Acetonitrile
117. D03015
118. Ab00053273_04
119. Ab00053273_05
120. A836855
121. Sr-05000001974
122. Q-200661
123. Q2775914
124. Sr-05000001974-1
125. 1-(4-methoxyphenyl)-3-(4-tert-butylphenyl)propane-1,3-dione
126. 1-(4-tert-butylphenyl)-3-(4-methoxyphenyl)-propane-1,3-dione
127. 1-(4-tert-butylphenyl)-3-(4-methoxyphenyl)propan-1,3-dione
128. Avobenzone, United States Pharmacopeia (usp) Reference Standard
129. Avobenzone, Pharmaceutical Secondary Standard; Certified Reference Material
| Molecular Weight | 310.4 g/mol |
|---|---|
| Molecular Formula | C20H22O3 |
| XLogP3 | 4.8 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 6 |
| Exact Mass | 310.15689456 g/mol |
| Monoisotopic Mass | 310.15689456 g/mol |
| Topological Polar Surface Area | 43.4 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 405 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Daily use of a sunscreen with a high SPF (greater than 15) on usually exposed skin is recommended for residents of areas of high ... /solar radiation/ who work outdoors or ... /enjoy/ regular outdoor recreation. Daily use of a sunscreen can reduce the cumulative ... /solar/ exposure that causes actinic keratoses and squamous-cell carcinoma.
IARC Working Group on the Evaluation of Cancer-Preventive Agents (2001) Sunscreens (IARC Handbooks of Cancer Prevention, Vol. 5), Lyon, IARC; Unit of Chemoprevention: Cancer-Preventive Effects of Sunscreens.
Sunscreen agents are indicated for the prevention of sunburn. In addition to limiting the skin's exposure to the sun, using sunscreen agents regularly when in the sun may help reduce long-term sun damage such as premature aging of the skin and skin cancer. /Sunscreen agents, topical; Included in US product labeling/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.
Sunscreen preparations should be applied uniformly and generously to all exposed skin surfaces, including lips, before exposure to UVB radiation. Two applications of the sunscreen may be needed for maximum protection. PABA-containing sunscreens are most effective when applied 1-2 hours before exposure to sunlight. Sunscreen products that are not water resistant should be reapplied after swimming, towel-drying, or profuse sweating and, because most sunscreens are easily removed from the skin, reapplication every 1-2 hours or according to the manufacturer's directions usually is required to provide adequate protection from UVB light. /Sunscreens/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
The manufacturers of sunscreen preparations with propellants warn that concentrating and subsequently inhaling the fumes from these preparations may be harmful or fatal. /Propellants/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Because the absorptive characteristics of skin of children younger than 6 months of age may differ from those of adults and because the immaturity of metabolic and excretory pathways of these children may limit their ability to eliminate any percutaneously absorbed sunscreen agent, sunscreen products should be used in children younger than 6 months of age only as directed by a clinician. It is possible that the characteristics of geriatric skin also differ from those of skin in younger adults, but these characteristics and the need for special considerations regarding use of sunscreen preparations in this age group are poorly understood. /Sunscreens/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Little information is available regarding the safety of chronic sunscreen usage, but commercially available physical and chemical sunscreens appear to have a low incidence of adverse effects. Derivatives of PABA, benzophenone, cinnamic acid, and salicylate and 2-phenylbenzimidazole-5-sulfonic acid have caused skin irritation including burning, stinging, pruritus, and erythema on rare occasions. /Sunscreens/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
Sunscreens should not be used as a means of extending the duration of solar exposure, such as prolonging sunbathing, and should not be used as a substitute for clothing on usually unexposed sites, such as the trunk and buttocks. /Sunscreens/
IARC Working Group on the Evaluation of Cancer-Preventive Agents (2001) Sunscreens (IARC Handbooks of Cancer Prevention, Vol. 5), Lyon, IARC; Unit of Chemoprevention: Cancer-Preventive Effects of Sunscreens.
For more Drug Warnings (Complete) data for AVOBENZONE (11 total), please visit the HSDB record page.
Sun protection factor, added in the sunscreen products for its wide spectrum ultraviolet absorption properties.
Sunscreening Agents
Chemical or physical agents that protect the skin from sunburn and erythema by absorbing or blocking ultraviolet radiation. (See all compounds classified as Sunscreening Agents.)
Solvents used in sunscreen products affect the stability and binding of the drug to the skin; in general, alcoholic solvents allow for the most rapid and deepest epidermal penetration of sunscreens. It appears that sunscreen agents are absorbed by the intact epidermis to varying degrees. /Sunscreens/
American Society of Health-System Pharmacists 2013; Drug Information 2013. Bethesda, MD. 2013
It blocks UVA I, UVA II, and UVB wavelengths, thereby limiting the impact of UV rays on skin. Diminish the penetration of ultraviolet (UV) light through the epidermis by absorbing UV radiation within a specific wavelength range. The amount and wavelength of UV radiation absorbed are affected by the molecular structure of the sunscreen agent.
Diminish the penetration of ultraviolet (UV) light through the epidermis by absorbing UV radiation within a specific wavelength range. The amount and wavelength of UV radiation absorbed are affected by the molecular structure of the sunscreen agent. /Sunscreen agents, topical/
Thomson/Micromedex. Drug Information for the Health Care Professional. Volume 1, Greenwood Village, CO. 2006.






