



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
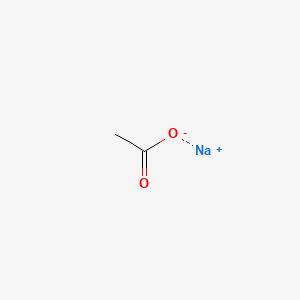

1. Sodium Acetate Trihydrate
2. Sodium Acetate, Anhydrous
1. 127-09-3
2. Acetic Acid, Sodium Salt
3. Sodium Acetate Anhydrous
4. Acetic Acid Sodium Salt
5. Sodium Acetate, Anhydrous
6. Anhydrous Sodium Acetate
7. Sodium Ethanoate
8. Fema No. 3024
9. Sodium-acetate
10. Acetic Acid, Sodium Salt (1:1)
11. Sodium;acetate
12. Mfcd00012459
13. Natriumacetat
14. Sodium Acetate,anhydrous
15. Nvg71zz7p0
16. Chebi:32954
17. Sodii Acetas
18. Natriumazetat
19. Nsc-77459
20. Natrium Aceticum
21. Octan Sodny [czech]
22. Caswell No. 741a
23. Natriumacetat [german]
24. Chembl1354
25. Fema Number 3024
26. Octan Sodny
27. Naoac
28. Hsdb 688
29. Sodium Acetate In Plastic Container
30. Einecs 204-823-8
31. Nsc 77459
32. Unii-nvg71zz7p0
33. Epa Pesticide Chemical Code 044006
34. Sodiumacetate
35. Sodium Aceate
36. Acona
37. Scfa
38. Ch3coona
39. Acetic Acidsodium Salt
40. Sodium Acetate Solution
41. Short Chain Fatty Acids
42. Sodium Acetate ,(s)
43. Ch3co2na
44. Dsstox_cid_7044
45. Ec 204-823-8
46. Dsstox_rid_78290
47. Sodium Acetate [mi]
48. Dsstox_gsid_27044
49. Sodium Acetate, Acs Reagent
50. Sodium Acetate [fhfi]
51. Sodium Acetate [hsdb]
52. Dtxsid2027044
53. Sodium Acetate [who-dd]
54. Sodium Acetate Solution, 0.3 M
55. Sodium Acetate, Biochemical Grade
56. Sodium Acetate Anhydrous Acs Usp
57. Tox21_202741
58. Sodium Acetate Anhydrous [ii]
59. Akos003052995
60. Akos015837569
61. Sodium Acetate, Bioxtra, >=99.0%
62. Db09395
63. Sodium Acetate, Reagentplus(r), 99%
64. Sodium Acetate, For Hplc, >=99.5%
65. Sodium Acetate,anhydrous [vandf]
66. Acetate, 1m Buffer Solution, Ph, 3.0
67. Acetate, 1m Buffer Solution, Ph, 3.5
68. Acetate, 1m Buffer Solution, Ph, 4.0
69. Acetate, 1m Buffer Solution, Ph, 5.0
70. Acetate, 1m Buffer Solution, Ph, 5.5
71. Ncgc00260289-01
72. Sodium Acetate, Ar, Anhydrous, >=99%
73. Sodium Acetate, Lr, Anhydrous, >=98%
74. Cas-127-09-3
75. E262
76. Sodium Acetate, Acs Reagent, >=99.0%
77. B7296
78. Ft-0635282
79. Ft-0659959
80. Ft-0689166
81. S0559
82. Sodium Acetate Anhydrous, >99%, Fcc, Fg
83. Sodium Acetate Anhydrous [orange Book]
84. Sodium Acetate, 99.995% Trace Metals Basis
85. Sodium Acetate, Saj First Grade, >=98.0%
86. Sodium Acetate, Trace Metals Grade, 99.99%
87. Sodium Acetate Anhydrous Acs Grade 12kg
88. Sodium Acetate, Jis Special Grade, >=98.5%
89. Sodium Acetate, Vetec(tm) Reagent Grade, 98%
90. Sodium Acetate Anhydrous [usp Monograph]
91. A805637
92. Q339940
93. J-005463
94. Sodium Acetate, For Hplc, 99.0-101.0% (nt)
95. Sodium Acetate, Puriss., Anhydrous, >=98%, Powder
96. Sodium Acetate, Anhydrous, Reagentplus(r), >=99.0%
97. Sodium Acetate, Anhydrous, For Molecular Biology, >=99%
98. Sodium Acetate, For Electrophoresis, >=99%, Crystalline
99. Sodium Acetate, 1m Aqueous Solution, Ph 4.5, Rnase Free
100. Sodium Acetate, 3m Aqueous Solution, Ph 4.5, Autoclaved
101. Sodium Acetate, 3m Aqueous Solution, Ph 5.2, Autoclaved
102. Sodium Acetate, 3m Aqueous Solution, Ph 5.2, Rnase Free
103. Sodium Acetate, 3m Aqueous Solution, Ph 7.0, Autoclaved
104. Sodium Acetate, 3m Aqueous Solution, Ph 7.0, Rnase Free
105. Sodium Acetate, Meets Usp Testing Specifications, Anhydrous
106. Sodium Acetate, United States Pharmacopeia (usp) Reference Standard
107. Sodium Acetate Solution, Bioultra, For Molecular Biology, ~3 M In H2o
108. Sodium Acetate, Bioultra, For Luminescence, Anhydrous, >=99.0% (nt)
109. Sodium Acetate, Puriss. P.a., Acs Reagent, Reag. Ph. Eur., Anhydrous
110. Sodium Acetate, Anhydrous, Bioultra, For Luminescence, For Molecular Biology, >=99.0% (nt)
111. Sodium Acetate, Anhydrous, Free-flowing, Redi-dri(tm), Acs Reagent, >=99.0%
112. Mettler-toledo Calibration Substance Me 30130599, Sodium Acetate Anhydrous, Tracable To Primary Standards (lgc)
113. Sodium Acetate Solution, Nmr Reference Standard, 50 Mm In D2o (99.9 Atom % D), Water 1 %, Nmr Tube Size 3 Mm X 8 In.
114. Sodium Acetate, Powder, Bioreagent, For Electrophoresis, Suitable For Cell Culture, Suitable For Insect Cell Culture, >=99%
| Molecular Weight | 82.03 g/mol |
|---|---|
| Molecular Formula | C2H3NaO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 82.00307362 g/mol |
| Monoisotopic Mass | 82.00307362 g/mol |
| Topological Polar Surface Area | 40.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 34.6 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Sodium acetate in plastic container |
| Active Ingredient | Sodium acetate anhydrous |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2meq/ml |
| Market Status | Prescription |
| Company | Hospira |
| 2 of 2 | |
|---|---|
| Drug Name | Sodium acetate in plastic container |
| Active Ingredient | Sodium acetate anhydrous |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | 2meq/ml |
| Market Status | Prescription |
| Company | Hospira |
Diuretics; Expectorants; Pharmaceutic Aids
National Library of Medicine's Medical Subject Headings online file (MeSH, 1999)
/FORMER USE/: HYOSCYAMUS SODIUM ACETATE, & PHENOBARBITAL ELIXIR COMBINES SMOOTH MUSCLE ANTISPASMODIC ACTION OF ... HYOSCYAMUS, WITH URINARY ALKALINIZING & DIURETIC EFFECTS OF SODIUM ACETATE, & SEDATIVE EFFECTS OF PHENOBARBITAL. /IT IS/ USED IN MANAGEMENT OF CYSTITIS & FOR BLADDER IRRITATION.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 86:00
DOSAGE OF ELIXIR OF HYOSCYAMUS CMPD /CONTAINING SODIUM ACETATE & PHENOBARBITAL/ IS 5 ML GIVEN 3 TIMES DAILY BEFORE MEALS.
American Hospital Formulary Service. Volumes I and II. Washington, DC: American Society of Hospital Pharmacists, to 1984., p. 86:00
... /SODIUM ACETATE/ HAS BEEN USED FOR PARENTERAL THERAPY OF ACIDOTIC CONDITIONS. IT IS BOTH A SYSTEMIC & URINARY ALKALIZER. /SODIUM ACETATE TRIHYDRATE, USP/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 772
For more Therapeutic Uses (Complete) data for SODIUM ACETATE (8 total), please visit the HSDB record page.
Injection, USP 40 mEq is indicated as a source of sodium, for addition to large volume intravenous fluids to prevent or correct hyponatremia in patients with restricted or no oral intake. It is also useful as an additive for preparing specific intravenous fluid formulas when the needs of the patient cannot be met by standard electrolyte or nutrient solutions. Sodium acetate and other bicarbonate precursors are alkalinising agents, and can be used to correct metabolic acidosis, or for alkalinisation of the urine.
Sodium is the principal cation of extracellular fluid. It comprises more than 90% of total cations at its normal plasma concentration of approximately 140 mEq/liter. The sodium ion exerts a primary role in controlling total body water and its distribution. Acetate ions acts as hydrogen ion acceptor which is alternative to bicarbonate.
B - Blood and blood forming organs
B05 - Blood substitutes and perfusion solutions
B05X - I.v. solution additives
B05XA - Electrolyte solutions
B05XA08 - Sodium acetate
Absorption
It is readily available in the circulation after IV administration.
Route of Elimination
Both the sodium and bicarbonate ions are excreted mainly in the urine. Some sodium is excreted in the feces, and small amounts may also be excreted in saliva, sweat, bile and pancreatic secretions.
In liver, sodium acetate is being metabolized into bicarbonate. To form bicarbonate, acetate is slowly hydrolyzed to carbon dioxide and water, which are then converted to bicarbonate by the addition of a hydrogen ion.
THE ACETATE ION IS RAPIDLY & COMPLETELY METABOLIZED BY BODY, & CONSEQUENTLY ADMIN OF SODIUM ACETATE IS EVENTUALLY EQUIVALENT TO GIVING SODIUM BICARBONATE. /SODIUM ACETATE TRIHYDRATE, USP/
Osol, A. and J.E. Hoover, et al. (eds.). Remington's Pharmaceutical Sciences. 15th ed. Easton, Pennsylvania: Mack Publishing Co., 1975., p. 772
CONSTANT RATE INFUSIONS OF SODIUM ACETATE GIVEN TO VOLUNTEERS, AT 4 MMOL/KG/HR INCR PLASMA ACETATE BY ONLY 0.41 MMOL/L, INDICATING A HIGH ACETATE METABOLIZING CAPACITY IN MAN.
KVEIM MH R ET AL; J OSLO CITY HOSP: 30 (8): 101 (1980)
Readily metabolized outside the liver.
American Medical Association. AMA Drug Evaluations Annual 1991. Chicago, IL: American Medical Association, 1991., p. 723
It works as a source of sodium ions especially in cases of hyponatremic patients. Sodium has a primary role in regulating extracellular fluid volume. It controls water distribution, fluid and electrolyte balance and the osmotic pressure of body fluids. Sodium is also involved in nerve conduction, muscle contraction, acid-base balance and cell nutrient uptake.






