



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
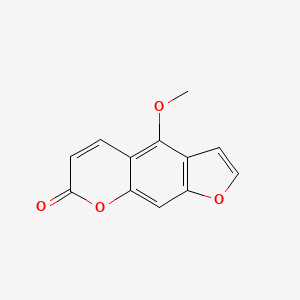

1. 5 Methoxy Psoralen
2. 5 Methoxypsoralen
3. 5-methoxy Psoralen
4. 5-methoxypsoralen
5. Pentaderm
1. 5-methoxypsoralen
2. 484-20-8
3. Bergaptene
4. Heraclin
5. Majudin
6. 4-methoxy-7h-furo[3,2-g]chromen-7-one
7. Bergaptan
8. Psoraderm
9. 5-mop
10. 5-methoxy Psoralen
11. O-methylbergaptol
12. Geralen
13. 5-methoxy-6,7-furanocoumarin
14. 5-methoxyfuranocoumarin
15. 4-methoxyfuro[3,2-g]chromen-7-one
16. Pentaderm
17. 7h-furo[3,2-g][1]benzopyran-7-one, 4-methoxy-
18. 4-methoxy-7h-furo(3,2-g)(1)benzopyran-7-one
19. 4-methoxyfuro[3,2-g]benzopyran-7-one
20. 5-methoxypsoralene
21. Nsc 95437
22. Bergaptene (dcf)
23. 4-methoxy-7h-furo[3,2-g][1]benzopyran-7-one
24. 5-methoxypsoralen With Ultraviolet A Therapy
25. Nsc95437
26. 6-hydroxy-4-methoxy-5-benzofuranacrylic Acid, Gamma-lactone
27. Nsc-95437
28. 4-methoxy-furo[3,2-g]chromen-7-one
29. 7h-furo(3,2-g)(1)benzopyran-7-one, 4-methoxy-
30. 4-methoxyfuro[3,2-g]benzopyrane-7-one
31. Chembl24171
32. 4fvk84c92x
33. Dsstox_cid_5560
34. Dsstox_rid_77830
35. Dsstox_gsid_25560
36. 5 Methoxypsoralen
37. Cas-484-20-8
38. Smr000112435
39. Ccris 4348
40. Hsdb 3466
41. Sr-05000002173
42. Einecs 207-604-5
43. Brn 0019560
44. Unii-4fvk84c92x
45. Pentaderm (tn)
46. 5-methoxy-psoralen
47. Mfcd00010272
48. 5-methoxyfurano[3,2-g]chromen-2-one
49. Spectrum_000794
50. 5-methoxy-2h-furo[3,2-g]chromen-2-one
51. Bergapten [mi]
52. Spectrum2_000534
53. Spectrum3_000663
54. Spectrum4_001478
55. Spectrum5_000155
56. 5-methoxypsoralen, 99%
57. 5-methoxypsoralen;heraclin
58. Bmse000758
59. 5-methoxypsoralen (obsol.)
60. 7h-furo[3, 4-methoxy-
61. Oprea1_562364
62. Schembl50066
63. Bspbio_002325
64. Kbiogr_002055
65. Kbioss_001274
66. Spectrum300546
67. 5-19-06-00004 (beilstein Handbook Reference)
68. Mls002207272
69. Mls002454380
70. Bergapten, Analytical Standard
71. Divk1c_000529
72. Methoxsalen Related Compound A
73. Spbio_000547
74. Megxp0_000990
75. Dtxsid1025560
76. Acon0_000984
77. Acon1_001979
78. Chebi:18293
79. Hms501k11
80. Kbio1_000529
81. Kbio2_001274
82. Kbio2_003842
83. Kbio2_006410
84. Kbio3_001545
85. Zinc57731
86. 5-methoxypsoralen [iarc]
87. Ninds_000529
88. Hms1923g13
89. Hms2268m24
90. Hms3652f19
91. Pharmakon1600-00300546
92. 5-methoxypsoralen [mart.]
93. 5-methoxypsoralen [who-dd]
94. Bcp30865
95. Hy-n0370
96. Tnp00299
97. Tox21_202357
98. Tox21_303255
99. Bdbm50067880
100. Ccg-39946
101. Nsc755877
102. S4239
103. Stk333038
104. Akos000276715
105. Db12216
106. Ds-2970
107. Nsc-755877
108. Sdccgmls-0066492.p001
109. Idi1_000529
110. Ncgc00017357-01
111. Ncgc00017357-02
112. Ncgc00017357-03
113. Ncgc00017357-04
114. Ncgc00017357-05
115. Ncgc00017357-06
116. Ncgc00017357-07
117. Ncgc00017357-08
118. Ncgc00091582-01
119. Ncgc00091582-02
120. Ncgc00091582-03
121. Ncgc00091582-04
122. Ncgc00178705-01
123. Ncgc00178705-02
124. Ncgc00256998-01
125. Ncgc00259906-01
126. Ac-20189
127. Ac-34208
128. Nci60_042121
129. Sbi-0051583.p002
130. Db-051552
131. 4-methoxy-7h-furo[3,2-g]benzopyran-7-one
132. B2840
133. Ft-0603416
134. N1304
135. Sw220008-1
136. 4-methoxy-7h-furo[3,2-g]chromen-7-one #
137. C01557
138. D07521
139. Methoxsalen Related Compound A [usp-rs]
140. Ab00052148_06
141. Ab00052148_07
142. 484b208
143. A827532
144. Q414779
145. Q-100536
146. Sr-05000002173-2
147. Sr-05000002173-3
148. Sr-05000002173-5
149. Brd-k12968785-001-02-6
150. Brd-k12968785-001-03-4
151. Brd-k12968785-001-06-7
152. Brd-k12968785-001-11-7
153. Methoxsalen Related Compound A [usp Impurity]
154. 6-hydroxy-4-methoxy-5-benzofuranacrylic Acid, .gamma.-lactone
| Molecular Weight | 216.19 g/mol |
|---|---|
| Molecular Formula | C12H8O4 |
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 1 |
| Exact Mass | 216.04225873 g/mol |
| Monoisotopic Mass | 216.04225873 g/mol |
| Topological Polar Surface Area | 48.7 Ų |
| Heavy Atom Count | 16 |
| Formal Charge | 0 |
| Complexity | 325 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/EXPL THER/ BACKGROUND: Psoralen plus ultraviolet (UV) A (PUVA) is the standard treatment for early stage mycosis fungoides (MF). When 8-methoxypsoralen (8-MOP) is used in PUVA therapy, it often produces intolerance reactions such as nausea, vomiting and headache. OBJECTIVES: To investigate whether 5-methoxypsoralen (5-MOP) is a safe and effective alternative to 8-MOP in PUVA therapy for MF. METHODS: A retrospective database search and chart review was done to identify patients with MF who received PUVA with either 5-MOP or 8-MOP as initial monotherapy at our institution. Between 1990 and 2004, 14 patients [seven men and seven women; mean age 70 years, range 51-82; National Cancer Institute disease stages IA (n = 6) and IB (n = 8)] received 5-MOP, and 24 patients [21 men and three women; mean age 58 years, range 28-89; disease stages IA (n = 11), IB (n = 12) and IIB (n = 1)] received 8-MOP. RESULTS: Twelve of 14 patients (86%) in the 5-MOP group and 22 of 24 (92%) in the 8-MOP group had a complete response to PUVA. These two subgroups of complete responders did not differ significantly in terms of PUVA therapy duration, number of treatments or cumulative UVA dose. They also did not differ significantly in terms of relapse-free rate [8% (one of 12) vs. 23% (five of 22)] or time to relapse [17 months (range 4-31) vs. 14 months (range 4-33)]. Moreover, PUVA maintenance therapy with either 5-MOP or 8-MOP in a subset of patients [26% (nine of 34)] did not affect long-term relapse-free status either. CONCLUSIONS: 5-MOP and 8-MOP have comparable therapeutic efficacy when used in PUVA therapy for MF.
PMID:16445785 Wackernagel A et al; Br J Dermatol 154 (3): 519-23 (2006)
5-Methoxypsoralen, in combination with UVA, is commonly used as a photochemotherapeutic agent for the treatment of psoriasis ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V40 328 (1986)
BACKGROUND: After oral intake, 5-methoxypsoralen (5-MOP) is as effective as 8-MOP for PUVA therapy for psoriasis, with a lower incidence of acute cutaneous side effects. OBJECTIVE: We compared bath-water delivery of 5-MOP and 8-MOP for photochemotherapy of psoriasis. METHODS: Twenty-two patients underwent phototesting with 0.0003% 5-MOP or 8-MOP aqueous solutions. Twelve patients with palmar psoriasis were studied with a side-to-side comparison, and 10 patients with recurrent plaque-type psoriasis were treated with one therapy or the other. RESULTS: Minimal phototoxic dose (MPD) values were 2.8 +/- 1.2 J/sq cm with 8-MOP and 2.0 +/- 1.2 J/sq cm with 5-MOP (p < 0.01). Both therapies cleared palmar lesions but 8-MOP required more UVA irradiation (46.3 +/- 21.0 J/sq cm vs 30.2 +/- 21.5 J/sq cm; p < 0.01) and more exposures (21.0 +/- 6.0 vs 17.0 +/- 5.0; p = 0.02). Bath-5-MOP-UVA was also more effective in the treatment of plaque-type psoriasis (cumulative UVA doses, 56.8 +/- 39.2 vs 59.1 +/- 27.9 J/sq cm; number of exposures, 20.0 +/- 5.7 vs 21.6 +/- 4.7), but these differences were not significant (p = NS). Patients developed an intense tan significantly earlier with 5-MOP than with 8-MOP (3.5 +/- 0.5 weeks vs 4.4 +/- 0.5 weeks; p < 0.01). CONCLUSION: Bath-5-MOP-UVA was more phototoxic than bath-8-MOP-UVA. It was more effective in the treatment of palmar psoriasis, whereas its greater pigmentogenic activity appeared to have an adverse effect on therapeutic effectiveness in the treatment of plaque-type psoriasis.
PMID:9204060 Calzavara-Pinton PG et al; J Am Acad Dermatol 36 (6 Pt 1): 945-9 (1997)
5-Methoxypsoralen, a naturally occurring linear furocoumarin, has been successfully used in combination with ultraviolet (UV) A irradiation [psoralen plus UV (PUVA)] to manage psoriasis and vitiligo. In patients and volunteers, PUVA 5-methoxypsoralen causes a dose-related increase in cutaneous photosensitivity. However, mean minimum phototoxic doses (MPD) were 30 to 50% greater with 5-methoxypsoralen than with 8-methoxypsoralen within individuals; this suggests lower photoactivity with 5-methoxypsoralen. In comparative clinical trials of parallel design, psoriasis clearance rates of > 90% or > 97% were observed in similar numbers of patients (60 to 77%) receiving oral PUVA 5-methoxypsoralen (typically 1.2 mg/kg) or oral PUVA 8-methoxypsoralen (0.6 mg/kg) treatment. Generally, 5-methoxypsoralen recipients required a greater total UVA exposure than 8-methoxypsoralen recipients to achieve end-point. However, study end-point was achieved sooner with oral or topical PUVA 5-methoxypsoralen in a small number of patients with psoriasis who received both treatments simultaneously and contralaterally. Up to 56% of patients with vitiligo achieved > 75% repigmentation with 5-methoxypsoralen (oral or topical) combined with UV irradiation (lamp or sun); the face and trunk were the most responsive areas. Lack of response to PUVA 5-methoxypsoralen treatment was observed in up to 16% of patients with psoriasis and, in 1 trial, in 22% of those with vitiligo. Lesion spreading during treatment of vitiligo was also observed in 7 (19%) patients in 1 study. The incidence and severity of adverse events was generally lower in PUVA 5-methoxypsoralen 1.2 mg/kg than in PUVA 8-methoxypsoralen 0.6 mg/kg recipients. Nausea and/or vomiting, pruritus and erythema were the most commonly reported adverse events in the short term; they occurred about 2 to 11 times more frequently in 8-methoxypsoralen than 5-methoxypsoralen recipients within clinical trials. Adverse hepatic events after oral administration of the drug were uncommon. Long term tolerability data for PUVA 5-methoxypsoralen are scarce; however, carcinogenicity was not reported during a 14-year observation period of 413 patients with psoriasis. CONCLUSION: Similar lesion clearance rates were observed with oral 5- or 8-methoxypsoralen plus UVA exposure in patients with vitiligo or psoriasis, although patients given 5-methoxypsoralen often required a greater total UV exposure than 8-methoxypsoralen recipients. The incidence of short term cutaneous and gastrointestinal adverse effects is markedly less with 5-methoxypsoralen than with 8-methoxypsoralen, which is an advantage, although the long term tolerability of 5-methoxypsoralen has yet to be fully established. Nevertheless, in appropriately selected patients, PUVA 5-methoxypsoralen therapy may be recommended as an alternative first-line systemic treatment option for the management of vitiligo or psoriasis.
PMID:9806110 McNeely W, Goa KL; Drugs 56 (4): 667-90 (1998)
5-Methoxypsoralen has been reported to be the single active agent in berloque dermatitis, causing patchy hyperpigmentation of the face and neck ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V40 337 (1986)
Photosensitizing Agents
Drugs that are pharmacologically inactive but when exposed to ultraviolet radiation or sunlight are converted to their active metabolite to produce a beneficial reaction affecting the diseased tissue. These compounds can be administered topically or systemically and have been used therapeutically to treat psoriasis and various types of neoplasms. (See all compounds classified as Photosensitizing Agents.)
Anti-Inflammatory Agents
Substances that reduce or suppress INFLAMMATION. (See all compounds classified as Anti-Inflammatory Agents.)
Dermatologic Agents
Drugs used to treat or prevent skin disorders or for the routine care of skin. (See all compounds classified as Dermatologic Agents.)
D - Dermatologicals
D05 - Antipsoriatics
D05B - Antipsoriatics for systemic use
D05BA - Psoralens for systemic use
D05BA03 - Bergapten
Micronized bergapten in capsules was absorbed slowly by volunteers (time to max serum concn 3.2 hr; elim half-time about 1 hr). When injected iv into rabbits, elim half-time 1-2 min (alpha-phase) & 15 min (beta-phase).
STOLK LM L; PHARM WEEKBL 117 (28): 609 (1982)
5-Methoxypsoralen showed a high binding affinity to serum proteins & 98-99% was protein bound. Its high binding affinity resulted in higher tissue concn. In the epidermis, it appeared to be bound to independent & noninteracting sites.
PMID:534612 ARTUC M ET AL; BR J DERMATOL 101 (6): 669 (1979)
Enrichment in epidermis of 5-methoxypsoralen was measured. It was concentrated by human epidermis & concn reached within the tissue was 10-500 times higher than concn of substance in surrounding buffer. The partitioning distribution among tissue components could account for its behavior.
PMID:7425669 ARTUC M ET AL; ARCH DERMATOL RES 268 (2): 129 (1980)
In young adult Hartley guinea-pigs, a linear relation was found between serum and epidermal concn of 5-methoxypsoralen, and the observed skin phototoxicity correlated with the serum 5-methoxypsoralen concn ... .
IARC. Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Geneva: World Health Organization, International Agency for Research on Cancer, 1972-PRESENT. (Multivolume work). Available at: https://monographs.iarc.fr/ENG/Classification/index.php, p. V40 336 (1986)
For more Absorption, Distribution and Excretion (Complete) data for 5-Methoxypsoralen (11 total), please visit the HSDB record page.
PURPOSE: To discuss the contribution of psoralen and bergapten metabolites on psoralens toxicity. METHODS: Computational chemistry prediction of metabolic reactions and toxicophoric groups based on the expert systems Derek and Meteor. RESULTS: a total of 15 metabolites were suggested for both psoralen and bergapten based on phase 1 and 2 biotransformations until the 3rd generation. Five toxicophoric substructures were shared among psoralen, bergapten and their corresponding metabolites; one toxicophoric marker (resorcinol) was only identified in bergapten and its biotransformation products. CONCLUSION: Although the toxic effects of psoralens are well known and documented, there is little information concerning the role of their metabolites in this process. We believe this work add to the knowledge of which molecular substructures are relevant to the process of metabolism and toxicity induction, thus guiding the search and development of more effective and less toxic drugs to treat vitiligo.
PMID:20067712 da Silva VB et al; J Pharm Pharm Sci 12 (3): 378-87 (2009)
A number of studies have demonstrated that cytochrome P450 (P450) converts furanocoumarin derivatives into reactive molecules, which form covalent bonds to biomolecules. 5-Methoxypsoralen (5-MOP) is a natural furanocoumarin from apiaceous plants. In this study, we examined the effect on 5-MOP metabolism of single nucleotide polymorphisms (SNPs) in CYP2A13. We used Escherichia coli-generated recombinant enzymes of wild-type CYP2A13*1 and five variants, CYP2A13*4 (R101Q), CYP2A13*5 (F453Y), CYP2A13*6 (R494C), CYP2A13*8 (D158E), and CYP2A13*9 (V323L). In high-performance liquid chromatography analyses of 5-MOP metabolic products, CYP2A13*1 converted 5-MOP into 5-MOP dihydrodiol; K(m) and V(max) values of the reaction were 1.44 +/- 0.17 uM and 4.23 +/- 0.36 nmol/(min x nmol P450), respectively. The generation of a dihydrodiol from 5-MOP implies that conversion by CYP2A13 causes toxicity due to the formation of covalent bonds with DNA or proteins. Most of the CYP2A13 variants could metabolize 5-MOP; K(m) values for CYP2A13*5, *6, *8, and *9 were 1.63 =/- 0.12, 1.36 +/- 0.10, 0.85 +/- 0.09, and 0.58 +/- 0.06 uM, respectively, and V(max) values were 3.20 +/- 0.13, 4.69 +/- 0.13, 2.34 +/- 0.07, and 1.84 +/- 0.09 nmol/(min x nmol P450), respectively. However, the processing of 5-MOP by CYP2A13*4 was not detectable. Based on this data, we hypothesize that SNPs within the CYP2A13 gene affect metabolism of 5-MOP in humans.
PMID:20798279 Goto T et al; Drug Metab Dispos 38 (12): 2110-6 (2010)
Bergapten has known human metabolites that include Unii-3abk64HG9O.
S73 | METXBIODB | Metabolite Reaction Database from BioTransformer | DOI:10.5281/zenodo.4056560
Micronized bergapten in capsules was absorbed slowly by volunteers (time to max serum concn 3.2 hr; elim half-time about 1 hr).
STOLK LM L; PHARM WEEKBL 117 (28): 609 (1982)
When injected iv into rabbits, elim half-time 1-2 min (alpha-phase) & 15 min (beta-phase).
STOLK LM L; PHARM WEEKBL 117 (28): 609 (1982)


