



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers
0
0

USA (Orange Book)

Europe

Canada
0

Australia

South Africa
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
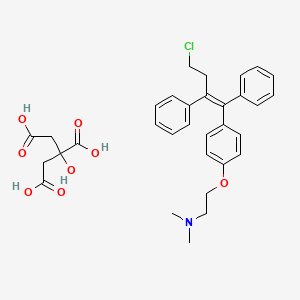

1. Citrate, Toremifene
2. Fareston
3. Fc 1157a
4. Fc-1157a
5. Fc1157a
6. Toremifene
7. Toremifene Citrate (1:1)
8. Toremifene, (e)-isomer
1. 89778-27-8
2. Fareston
3. Toremifene Citrate [usan]
4. Toremifene (citrate)
5. Fc 1157a
6. Nk 622
7. Nsc 613680
8. Fc-1157a
9. 89778-27-8 (citrate)
10. Nsc-613680
11. Nsc613680
12. 2-(p-((z)-4-chloro-1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethylethylamine Citrate (1:1)
13. 2498y783qt
14. Toremifene Citrate (fareston, Acapodene)
15. Dsstox_cid_1367
16. Dsstox_rid_76113
17. Dsstox_gsid_21367
18. (z)-2-(4-(4-chloro-1,2-diphenylbut-1-en-1-yl)phenoxy)-n,n-dimethylethan-1-amine 2-hydroxypropane-1,2,3-tricarboxylate
19. Ethanamine, 2-(4-(4-chloro-1,2-diphenyl-1-butenyl)phenoxy)-n,n-dimethyl-, (z)-, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
20. (2-{4-[(1z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenoxy}ethyl)dimethylamine; 2-hydroxypropane-1,2,3-tricarboxylic Acid
21. 2-({4-[(1z)-4-chloro-1,2-diphenylbut-1-en-1-yl]phenyl}oxy)-n,n-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate (salt)
22. Cas-89778-27-8
23. Ccris 6719
24. Unii-2498y783qt
25. 2-[p-[(z)-4-chloro-1,2-diphenyl-1-butenyl]phenoxy]-n,n-dimethylethylamine Citrate (1:1)
26. Cpd000469213
27. Schembl4564
28. (z)-4-chloro-1,2-diphenyl-1-(4-(2-(n,n-dimethylamino)ethoxy)phenyl)-1-butene Citrate (1:1)
29. Mls001306432
30. Mls006011608
31. Chebi:9636
32. Toremifene Citrate [mi]
33. Chembl1200675
34. Dtxsid2021367
35. Toremifene Citrate (jan/usan)
36. Toremifene Citrate [jan]
37. Hms2052c03
38. Hms2230p09
39. Hms3264l11
40. Pharmakon1600-01505682
41. Toremifene Citrate [vandf]
42. Toremifene Citrate [mart.]
43. Hy-b0005
44. Toremifene Citrate [who-dd]
45. Tox21_111877
46. Tox21_301740
47. Mfcd01729016
48. Nsc759190
49. S1776
50. Akos015888270
51. Ac-1985
52. Ccg-101072
53. Cs-1272
54. Ks-5242
55. Nc00322
56. Nsc-759190
57. 2-[4-[(z)-4-chloro-1,2-diphenyl-but-1-enyl]phenoxy]-n,n-dimethyl-ethanamine; Citric Acid
58. Toremifene Citrate [orange Book]
59. Ncgc00255310-01
60. 2-[4-[(z)-4-chloro-1,2-diphenylbut-1-enyl]phenoxy]-n,n-dimethylethanamine; 2-hydroxypropane-1,2,3-tricarboxylic Acid
61. Smr004703372
62. Toremifene Citrate Salt, >=98% (hplc)
63. Sw197702-3
64. T2832
65. D00967
66. 778t267
67. A843307
68. Sr-01000763502-3
69. Q27253831
70. 2-[p-[(z)-4-chloro-1,n- Dimethylethylamine Citrate (1:1)
71. (z)-2-[4-(4-chloro-1,2-diphenyl-1-butenyl)phenoxy]-n,n-dimethylethanamine Citrate
72. (z)-2-[4-(4-chloro-1,n- Dimethylethanamine, 2-hydroxy-1,2,3-propanetricarboxylate (1:1)
73. (z)-4-chloro-1-(4-dimethylaminoethoxyphenyl)-1,2-diphenyl-1-butene Citrate
74. (z)-2-(4-(4-chloro-1,2-diphenylbut-1-en-1-yl)phenoxy)-n,n-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate
75. (z)-2-(4-(4-chloro-1,2-diphenylbut-1-enyl)phenoxy)-n,n-dimethylethanamine 2-hydroxypropane-1,2,3-tricarboxylate
76. 2-[4-[(z)-4-chloranyl-1,2-diphenyl-but-1-enyl]phenoxy]-n,n-dimethyl-ethanamine; 2-oxidanylpropane-1,2,3-tricarboxylic Acid
| Molecular Weight | 598.1 g/mol |
|---|---|
| Molecular Formula | C32H36ClNO8 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 14 |
| Exact Mass | 597.2129448 g/mol |
| Monoisotopic Mass | 597.2129448 g/mol |
| Topological Polar Surface Area | 145 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 710 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
First line hormone treatment of hormone-dependent metastatic breast cancer in postmenopausal patients.
Fareston is not recommended for patients with estrogen receptor negative tumours.
Antineoplastic Agents, Hormonal
Antineoplastic agents that are used to treat hormone-sensitive tumors. Hormone-sensitive tumors may be hormone-dependent, hormone-responsive, or both. A hormone-dependent tumor regresses on removal of the hormonal stimulus, by surgery or pharmacological block. Hormone-responsive tumors may regress when pharmacologic amounts of hormones are administered regardless of whether previous signs of hormone sensitivity were observed. The major hormone-responsive cancers include carcinomas of the breast, prostate, and endometrium; lymphomas; and certain leukemias. (From AMA Drug Evaluations Annual 1994, p2079) (See all compounds classified as Antineoplastic Agents, Hormonal.)
Bone Density Conservation Agents
Agents that inhibit BONE RESORPTION and/or favor BONE MINERALIZATION and BONE REGENERATION. They are used to heal BONE FRACTURES and to treat METABOLIC BONE DISEASES such as OSTEOPOROSIS. (See all compounds classified as Bone Density Conservation Agents.)
Selective Estrogen Receptor Modulators
A structurally diverse group of compounds distinguished from ESTROGENS by their ability to bind and activate ESTROGEN RECEPTORS but act as either an agonist or antagonist depending on the tissue type and hormonal milieu. They are classified as either first generation because they demonstrate estrogen agonist properties in the ENDOMETRIUM or second generation based on their patterns of tissue specificity. (Horm Res 1997;48:155-63) (See all compounds classified as Selective Estrogen Receptor Modulators.)
L02BA02






