



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
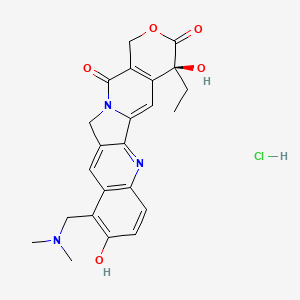

1. 9 Dimethylaminomethyl 10 Hydroxycamptothecin
2. 9-dimethylaminomethyl-10-hydroxycamptothecin
3. Hycamtamine
4. Hycamtin
5. Hydrochloride, Nogitecan
6. Hydrochloride, Topotecan
7. Nogitecan Hydrochloride
8. Nsc 609699
9. Nsc-609699
10. Nsc609699
11. Sk And F 104864 A
12. Sk And F-104864-a
13. Sk And F104864a
14. Skf 104864 A
15. Skf-104864-a
16. Skf104864a
17. Topotecan
18. Topotecan Monohydrochloride, (s)-isomer
1. 119413-54-6
2. Topotecan Hcl
3. Hycamtin
4. Nogitecan Hydrochloride
5. Topotecan (hydrochloride)
6. Topotecan Monohydrochloride
7. Sk&f S-104864-a
8. Evotopin
9. Topotecan Hydrochloride [usan]
10. Nsc 609699
11. Topotecan Teva
12. Topotecan Actavis
13. Topotecan Hospira
14. Nsc609699
15. Skf 104864a
16. Nsc-609699
17. Nsc-759263
18. Topotecan (as Hydrochloride)
19. (s)-10-((dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione Monohydrochloride
20. 956s425zcy
21. Sk&f-s-104864-a
22. Ncgc00095189-01
23. Hycamtin (tn)
24. Skf 104864a; Nsc 609669
25. Dsstox_cid_25952
26. Dsstox_rid_81248
27. Dsstox_gsid_45952
28. 1h-pyrano(3',4':6,7)indolizino(1,2-b)quinoline-3,14(4h,12h)-dione, 10-((dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-, Monohydrochloride, (s)-
29. Nogitecan Hydrochloride (jan)
30. Topotecan Hydrochloride (usan)
31. Skf 104864a (hydrochloride);nsc 609669 (hydrochloride)
32. Topotecanhydrochloride
33. Chembl1607
34. Hycamptamine Hydrochloride
35. Nogitecan Hydrochloride [jan]
36. (s)-10-((dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione Hydrochloride
37. Smr000466344
38. Cas-119413-54-6
39. Sk&f-s-104864a
40. Nsc 609669
41. Drg-0288
42. 9-dimethylaminomethyl-10-hydroxycamptothecin.
43. Potactasol
44. Evotropin
45. Unii-956s425zcy
46. Nogitecan Hcl
47. Potactasol (tn)
48. (s)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione Hydrochloride
49. Topotecan Hydrocholoride
50. Topotecan Hcl - Hycamtin
51. Schembl7247
52. Hycamtin (tn) (glaxosmith)
53. Mls000759456
54. Mls001401447
55. Spectrum1505820
56. Dtxsid1045952
57. Skfs 104864a
58. Hms1922h22
59. Pharmakon1600-01505820
60. 10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione Hydrochloride
61. Amy24931
62. Bcp00930
63. Skf-104864a
64. Skf-s 104864-a
65. Topotecan Hydrochloride [mi]
66. Tox21_111478
67. Ccg-37460
68. Hy-13768a
69. Mfcd00866235
70. Ncgc00014925
71. Nci609699
72. Nk-211
73. Nsc759263
74. S1231
75. Akos015900415
76. Sk&f 104864-a
77. Tox21_111478_1
78. Ac-1551
79. Cs-1498
80. Ks-1410
81. Nc00177
82. Nsc 759263
83. Topotecan Hydrochloride [mart.]
84. Topotecan Hydrochloride [vandf]
85. Topotecan Hydrochloride [usp-rs]
86. Topotecan Hydrochloride [who-dd]
87. Ncgc00014925-06
88. Ncgc00095189-02
89. Ncgc00095189-03
90. Sw197557-5
91. T-160
92. T2795
93. Topotecan Hydrochloride [orange Book]
94. D02168
95. 413t546
96. E-89/001
97. 9-dimethylaminomethyl-10-hydroxycamptothecin, Hcl Salt
98. Q27271753
99. 9-[(dimethylamino)methyl]-10-hydroxy-(20s)-camptothecin, Hcl
100. (4s)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4',6-7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione
101. (4s)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dionehydrochloride (1:1)
102. (s)-10-((dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dionehydrochloride
103. (s)-10-((dimethylamino)methyl)-4-ethyl-4,9-dihydroxy-1h-pyrano[3,4:6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dione Hydrochloride
104. (s)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[1,2-b]quinoline-3,14(4h,12h)-dionemonohydrochloride
105. (s)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1h-pyrano[3',4':6,7]indolizino[l,2-b]quinoline-3,14(4h,12h)-dione Monohydrochloride
106. 1h-pyrano[3',7]indolizino[1,2-b]quinoline- 3,14(4h,12h)-dione, 10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-, Monohydrochloride, (4s)-
| Molecular Weight | 457.9 g/mol |
|---|---|
| Molecular Formula | C23H24ClN3O5 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 3 |
| Exact Mass | 457.1404486 g/mol |
| Monoisotopic Mass | 457.1404486 g/mol |
| Topological Polar Surface Area | 103 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 867 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Topotecan hydrochloride |
| Drug Label | Topotecan hydrochloride is a semi-synthetic derivative of camptothecin and is an anti-tumor drug with topoisomerase I-inhibitory activity.The chemical name for topotecan hydrochloride is (S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[... |
| Active Ingredient | Topotecan hydrochloride |
| Dosage Form | Injectable; Solution |
| Route | Injection; Intravenous |
| Strength | eq 4mg base/4ml (eq 1mg base/ml); eq 4mg base/vial |
| Market Status | Prescription |
| Company | Hospira; Fresenius Kabi Oncol; Accord Hlthcare; Sun Pharm Inds; Innopharma Licensing; Teva Pharms Usa; Actavis Elizabeth; Fresenius Kabi Usa; Onco Therapies; Sagent Pharms; Dr Reddys Labs; Three Rivers Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Topotecan hydrochloride |
| Drug Label | Topotecan hydrochloride is a semi-synthetic derivative of camptothecin and is an anti-tumor drug with topoisomerase I-inhibitory activity.The chemical name for topotecan hydrochloride is (S)-10-[(dimethylamino)methyl]-4-ethyl-4,9-dihydroxy-1H-pyrano[... |
| Active Ingredient | Topotecan hydrochloride |
| Dosage Form | Injectable; Solution |
| Route | Injection; Intravenous |
| Strength | eq 4mg base/4ml (eq 1mg base/ml); eq 4mg base/vial |
| Market Status | Prescription |
| Company | Hospira; Fresenius Kabi Oncol; Accord Hlthcare; Sun Pharm Inds; Innopharma Licensing; Teva Pharms Usa; Actavis Elizabeth; Fresenius Kabi Usa; Onco Therapies; Sagent Pharms; Dr Reddys Labs; Three Rivers Pharms |
Hycamtin capsules are indicated as monotherapy for the treatment of adult patients with relapsed small cell lung cancer (SCLC) for whom re-treatment with the first-line regimen is not considered appropriate.
Topotecan is indicated for the treatment of patients with metastatic carcinoma of the ovary after failure of first-line or subsequent therapy.
Hycamtin capsules are indicated as monotherapy for the treatment of adult patients with relapsed small cell lung cancer (SCLC) for whom re-treatment with the first-line regimen is not considered appropriate.
Topotecan monotherapy is indicated for the treatment of patients with relapsed small-cell lung cancer (SCLC) for whom re-treatment with the first-line regimen is not considered appropriate.
Topotecan in combination with cisplatin is indicated for patients with carcinoma of the cervix recurrent after radiotherapy and for patients with stage IVB disease. Patients with prior exposure to cisplatin require a sustained treatment-free interval to justify treatment with the combination.
Topotecan monotherapy is indicated for the treatment of patients with relapsed small cell lung cancer [SCLC] for whom re-treatment with the first-line regimen is not considered appropriate.
Topotecan in combination with cisplatin is indicated for patients with carcinoma of the cervix recurrent after radiotherapy and for patients with Stage IVB disease. Patients with prior exposure to cisplatin require a sustained treatment free interval to justify treatment with the combination.
Topotecan monotherapy is indicated for the treatment of:
- patients with metastatic carcinoma of the ovary after failure of first-line or subsequent therapy
- patients with relapsed small cell lung cancer (SCLC) for whom re-treatment with thefirst-line regimen is not considered appropriate.
Topotecan in combination with cisplatin is indicated for patients with carcinoma of the cervix recurrent after radiotherapy and for patients with Stage IVB disease. Patients with prior exposure to cisplatin require a sustained treatment free interval to justify treatment with the combination.
Topotecan monotherapy is indicated for the treatment of:
- patients with metastatic carcinoma of the ovary after failure of first line or subsequent therapy;
- patients with relapsed small cell lung cancer [SCLC] for whom re-treatment with the first-line regimen is not considered appropriate.
Topotecan in combination with cisplatin is indicated for patients with carcinoma of the cervix recurrent after radiotherapy and for patients with Stage IVB disease. Patients with prior exposure to cisplatin require a sustained treatment free interval to justify treatment with the combination.
Topotecan monotherapy is indicated for the treatment of patients with relapsed small cell lung cancer (SCLC) for whom re-treatment with the first-line regimen is not considered appropriate.
Topotecan in combination with cisplatin is indicated for patients with carcinoma of the cervix recurrent after radiotherapy and for patients with Stage IVB disease. Patients with prior exposure to cisplatin require a sustained treatment free interval to justify treatment with the combination.
Topotecan is indicated for the treatment of patients with metastatic carcinoma of the ovary after failure of first-line or subsequent therapy.
Topoisomerase I Inhibitors
Compounds that inhibit the activity of DNA TOPOISOMERASE I. (See all compounds classified as Topoisomerase I Inhibitors.)
L01CE01
L01XX17
L01XX17
L01XX17
L01XX17
L01XX17
L01XX17






