



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Aggrastat
2. Agrastat
3. L 700,462
4. L 700462
5. L-700,462
6. L-700462
7. L700,462
8. Mk 383
9. Mk-383
10. N-(butylsulfonyl)-o-(4-(4-piperidyl)butyl)-l-tyrosine
11. Tirofiban
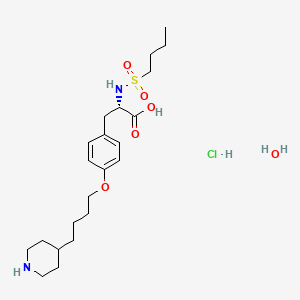
12. Tirofiban Hydrochloride Monohydrate
1. Tirofiban Hydrochloride Monohydrate
2. 150915-40-5
3. Tirofiban Hcl
4. Tirofiban Hydrochloride Hydrate
5. Aggrastat
6. Tirofiban (hydrochloride Monohydrate)
7. Tirofiban Hcl Hydrate
8. Tirofiban Hcl Monohydrate
9. 142373-60-2
10. Tirofiban Hydrochloride [usan]
11. Mk-383 Hydrochloride
12. Chebi:9606
13. Mk 383
14. Mk-383
15. 6h925f8o5j
16. L-700,462
17. Ncgc00182085-01
18. L 700462
19. Tirofibanhydrochloridemonohydrate
20. Dsstox_cid_28561
21. Dsstox_rid_82833
22. N-(butylsulfonyl)-4-(4-(4-piperidyl)butoxy)-l-phenylalanine Monohydrochloride Monohydrate
23. Dsstox_gsid_48635
24. Tirofiban Hydrochloride (usan)
25. L-700462
26. L-tyrosine, N-(butylsulfonyl)-o-(4-(4-piperidinyl)butyl)-, Monohydrochloride, Monohydrate
27. N-(butylsulfonyl)-o-(4-piperidin-4-ylbutyl)-l-tyrosine Hydrochloride Monohydrate
28. (2s)-2-(butylsulfonylamino)-3-[4-(4-piperidin-4-ylbutoxy)phenyl]propanoic Acid Hydrochloride Monohydrate
29. (s)-2-(butylsulfonamido)-3-(4-(4-(piperidin-4-yl)butoxy)phenyl)propanoic Acid Hydrochloride Hydrate
30. Cas-150915-40-5
31. Unii-6h925f8o5j
32. Aggrastat (tn)
33. 4-(4-pyridinyl)butanol Hydrochloride
34. Tirofiban, Hydrochloride
35. Schembl41325
36. Chembl3189072
37. Dtxsid5048635
38. Tirofibanhydrochloride Monohydrate
39. Act04424
40. Bcp06919
41. Tox21_112980
42. Mfcd07368623
43. S8594
44. Tox21_112980_1
45. Ccg-222411
46. Cs-0948
47. Tirofiban Hydrochloride [mart.]
48. Tirofiban Hydrochloride [vandf]
49. Ncgc00263584-01
50. (2s)-2-(butylsulfonylamino)-3-[4-[4-(4-piperidyl)butoxy]phenyl]propanoic Acid Hydrochloride Hydrate
51. As-11027
52. Bt164476
53. Hy-17369
54. Tirofiban Hydrochloride Monohydrate- Bio-x
55. Tirofiban Hydrochloride [orange Book]
56. D01029
57. Tirofiban Hydrochloride Monohydrate [mi]
58. 373t602
59. A908673
60. Q-101314
61. Tirofiban Hydrochloride Monohydrate [who-dd]
62. Tirofiban Hydrochloride Monohydrate, >=98% (hplc)
63. Q27108448
64. L-tyrosine, N-(butylsulfonyl)-o-(4-(4-piperidinyl)butyl)-, Hydrochloride, Hydrate (1:1:1)
| Molecular Weight | 495.1 g/mol |
|---|---|
| Molecular Formula | C22H39ClN2O6S |
| Hydrogen Bond Donor Count | 5 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 14 |
| Exact Mass | 494.2217358 g/mol |
| Monoisotopic Mass | 494.2217358 g/mol |
| Topological Polar Surface Area | 114 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 579 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
Platelet Aggregation Inhibitors
Drugs or agents which antagonize or impair any mechanism leading to blood platelet aggregation, whether during the phases of activation and shape change or following the dense-granule release reaction and stimulation of the prostaglandin-thromboxane system. (See all compounds classified as Platelet Aggregation Inhibitors.)
Fibrinolytic Agents
Fibrinolysin or agents that convert plasminogen to FIBRINOLYSIN. (See all compounds classified as Fibrinolytic Agents.)


