



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
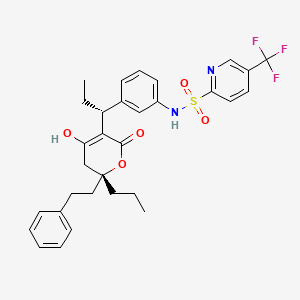

1. (2r)-3,6-dihydro-6-oxo-2-(2-phenylethyl)-2-propyl-5-((1r)-1-(3-(((5-(trifluoromethyl)-2-pyridinyl)sulfonyl)amino)phenyl)propyl)-2h-pyran-4-yl Beta-d-glucopyranosiduronic Acid
2. 14c-tipranavir
3. 3'-((1r)-1-((6r)-5,6-dihydro-4-hydroxy-2-oxo-6-phenethyl-6-propyl-2h-pyran-3-yl)propyl)-5-(trifluoromethyl)-2-pyridinesulfonanilide
4. Aptivus
5. C14-tipranavir
6. Pnu 140690
7. Pnu-140690
8. Pnu-140690e
9. Tipranavir C-14
10. Tipranavir Disodium
11. Tipranavir Glucuronide
12. Tipranavir Sodium
13. Tipranavir, (r-(r*,r*))-isomer
14. Tipranavir, (r-(r*,s*))-isomer
15. Tipranavir, (s-(r*,r*))-isomer
16. Tipranavir, (s-(r*,s*))-isomer
1. 174484-41-4
2. Aptivus
3. Pnu-140690
4. Tpv
5. Tipranavir (inn)
6. U-140690
7. Chebi:63628
8. Zzt404xd09
9. 3'-((1r)-1-((6r)-5,6-dihydro-4-hydroxy-2-oxo-6-phenethyl-6-propyl-2h-pyran-3-yl)propyl)-5-(trifluoromethyl)-2-pyridinesulfonanilide
10. 2-pyridinesulfonamide, N-[3-[(1r)-1-[(6r)-5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2h-pyran-3-yl]propyl]phenyl]-5-(trifluoromethyl)-
11. N-(3-{(1r)-1-[(6r)-4-hydroxy-2-oxo-6-phenethyl-6-propyl-5,6-dihydro-2h-pyran-3-yl]propyl}phenyl)-5-(trifluoromethyl)-2-pyridinesulfonamide
12. Tipranavir [inn]
13. N-(3-{(1r)-1-[(6r)-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-5,6-dihydro-2h-pyran-3-yl]propyl}phenyl)-5-(trifluoromethyl)pyridine-2-sulfonamide
14. Pnu 140690
15. Aptivus(tm)
16. N-[3-[(1r)-1-[(2r)-4-hydroxy-6-oxo-2-(2-phenylethyl)-2-propyl-3h-pyran-5-yl]propyl]phenyl]-5-(trifluoromethyl)pyridine-2-sulfonamide
17. Aptivus (tn)
18. Tipranavir [inn:ban]
19. Unii-zzt404xd09
20. Hsdb 8083
21. Ncgc00182028-01
22. 2-pyridinesulfonamide, N-(3-((1r)-1-((6r)-5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2h-pyran-3-yl)propyl)phenyl)-5-(trifluoromethyl)-
23. N-{3-[(1r)-1-[(6r)-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-5,6-dihydro-2h-pyran-3-yl]propyl]phenyl}-5-(trifluoromethyl)pyridine-2-sulfonamide
24. U 140690
25. 1d4y
26. 2o4l
27. 2o4n
28. 2o4p
29. Tipranavir [mi]
30. Tipranavir [vandf]
31. Tipranavir [mart.]
32. Dsstox_cid_28548
33. Dsstox_rid_82820
34. Tipranavir [who-dd]
35. Dsstox_gsid_48622
36. Schembl40629
37. Schembl40630
38. N-[3-[(1r)-1-[(2r)-6-hydroxy-4-oxo-2-phenethyl-2-propyl-3h-pyran-5-yl]propyl]phenyl]-5-(trifluoromethyl)pyridine-2-sulfonamide
39. Tipranavir [ema Epar]
40. Chembl183041
41. Chembl222559
42. Dtxsid6048622
43. Tipranavir [orange Book]
44. Ex-a4113
45. Tox21_112962
46. Bdbm50479982
47. Akos030254403
48. Zinc100016058
49. Zinc100022637
50. Cs-1210
51. Db00932
52. Ncgc00379087-01
53. Ncgc00379087-02
54. As-79082
55. Hy-15148
56. N-[3-[(1r)-1-[(2r)-6-hydroxy-4-oxo-2-(2-phenylethyl)-2-propyl-3h-pyran-5-yl]propyl]phenyl]-5-(trifluoromethyl)pyridine-2-sulfonamide
57. N-[3-[(1r)-1-[(6r)-5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2h-pyran-3-yl]propyl]phenyl]-5-(trifluoromethyl)-2-pyridinesulfonamide
58. Cas-174484-41-4
59. D08605
60. A811642
61. Q423404
62. J-010991
63. 2-pyridinesulfonamide, N-(3-(1-(5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2h-pyran-3-yl)propyl)phenyl)-5-(trifluoromethyl)-, (r-(r*,r*))-
64. N-[3-[(1r)-1-[(2r)-4-hydroxy-6-oxo-2-phenethyl-2-propyl-3h-pyran-5-yl]propyl]phenyl]-5-(trifluoromethyl)pyridine-2-sulfonamide
65. N-[3-[(1r)-1-[(2r)-6-hydroxy-4-oxo-2-(2-phenylethyl)-2-propyl-3h-pyran-5-yl]propyl]phenyl]-5-(trifluoromethyl)-2-pyridinesulfonamide
66. N-[3-[(1r)-1-[(2r)-6-oxidanyl-4-oxidanylidene-2-(2-phenylethyl)-2-propyl-3h-pyran-5-yl]propyl]phenyl]-5-(trifluoromethyl)pyridine-2-sulfonamide
| Molecular Weight | 602.7 g/mol |
|---|---|
| Molecular Formula | C31H33F3N2O5S |
| XLogP3 | 7 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 10 |
| Rotatable Bond Count | 11 |
| Exact Mass | 602.20622782 g/mol |
| Monoisotopic Mass | 602.20622782 g/mol |
| Topological Polar Surface Area | 114 Ų |
| Heavy Atom Count | 42 |
| Formal Charge | 0 |
| Complexity | 1050 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Aptivus |
| PubMed Health | Tipranavir (By mouth) |
| Drug Classes | Antiretroviral Agent, Antiviral |
| Drug Label | APTIVUS is a protease inhibitor of HIV-1 belonging to the class of 4-hydroxy-5,6-dihydro-2-pyrone sulfonamides.The chemical name of tipranavir is 2-Pyridinesulfonamide, N-[3-[(1R)-1-[(6R)-5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2H-pyra... |
| Active Ingredient | Tipranavir |
| Dosage Form | Capsule; Solution |
| Route | oral; Oral |
| Strength | 250mg; 100mg/ml |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
| 2 of 2 | |
|---|---|
| Drug Name | Aptivus |
| PubMed Health | Tipranavir (By mouth) |
| Drug Classes | Antiretroviral Agent, Antiviral |
| Drug Label | APTIVUS is a protease inhibitor of HIV-1 belonging to the class of 4-hydroxy-5,6-dihydro-2-pyrone sulfonamides.The chemical name of tipranavir is 2-Pyridinesulfonamide, N-[3-[(1R)-1-[(6R)-5,6-dihydro-4-hydroxy-2-oxo-6-(2-phenylethyl)-6-propyl-2H-pyra... |
| Active Ingredient | Tipranavir |
| Dosage Form | Capsule; Solution |
| Route | oral; Oral |
| Strength | 250mg; 100mg/ml |
| Market Status | Prescription |
| Company | Boehringer Ingelheim |
Anti-HIV Agents
National Library of Medicine's Medical Subject Headings online file (MeSH, 2012)
Tipranavir with low-dose ritonavir (ritonavir-boosted tipranavir) is used in conjunction with other antiretroviral agents for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults, adolescents, and pediatric patients 2 years of age and older with evidence of viral replication who are antiretroviral-experienced and infected with HIV-1 strains resistant to multiple HIV protease inhibitors (PIs). /Included in US product labeling/
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 687
/BOXED WARNING/ WARNING: HEPATOTOXICITY and INTRACRANIAL HEMORRHAGE. Hepatotoxicity: Clinical hepatitis and hepatic decompensation, including some fatalities, have been reported. Extra vigilance is warranted in patients with chronic hepatitis B or hepatitis C co-infection, as these patients have an increased risk of hepatotoxicity. Intracranial Hemorrhage: Both fatal and non-fatal intracranial hemorrhage have been reported.
US Natl Inst Health; DailyMed. Current Medication Information for APTIVUS (tipranavir) capsule, liquid filled APTIVUS (tipranavir) solution (April 2012). Available from, as of November 21, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=08982e49-d2eb-4b25-b01a-1be52fd669ef
New onset diabetes mellitus, exacerbation of pre-existing diabetes mellitus and hyperglycemia have been reported during post-marketing surveillance in HIV-1 infected patients receiving protease inhibitor therapy. Some patients required either initiation or dose adjustments of insulin or oral hypoglycemic agents for treatment of these events. In some cases, diabetic ketoacidosis has occurred. In those patients who discontinued protease inhibitor therapy, hyperglycemia persisted in some cases. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made and a causal relationship between protease inhibitor therapy and these events has not been established.
US Natl Inst Health; DailyMed. Current Medication Information for APTIVUS (tipranavir) capsule, liquid filled APTIVUS (tipranavir) solution (April 2012). Available from, as of November 25, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=08982e49-d2eb-4b25-b01a-1be52fd669ef
Aptivus should be used with caution in patients with a known sulfonamide allergy. Tipranavir contains a sulfonamide moiety. The potential for cross-sensitivity between drugs in the sulfonamide class and Aptivus is unknown.
US Natl Inst Health; DailyMed. Current Medication Information for APTIVUS (tipranavir) capsule, liquid filled APTIVUS (tipranavir) solution (April 2012). Available from, as of November 25, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=08982e49-d2eb-4b25-b01a-1be52fd669ef
Rash, including maculopapular rash, urticarial rash, and possible photosensitivity reaction, has been reported in patients receiving ritonavir-boosted tipranavir. Rash occurred in 10% of women, 8% of men, and 21% of children receiving ritonavir-boosted tipranavir in clinical studies. The median time to onset of rash was 53 days and the median duration of rash was 22 days in adults. Rash accompanied by joint pain or stiffness, throat tightness, or generalized pruritus also has been reported. Discontinue tipranavir if severe rash develops.
American Society of Health-System Pharmacists 2012; Drug Information 2012. Bethesda, MD. 2012, p. 688
For more Drug Warnings (Complete) data for Tipranavir (16 total), please visit the HSDB record page.
For combination antiretroviral treatment of HIV-1 infected adult patients with evidence of viral replication, who are highly treatment-experienced or have HIV-1 strains resistant to multiple protease inhibitors.
FDA Label
Aptivus, co-administered with low-dose ritonavir, is indicated for combination antiretroviral treatment of HIV-1 infection in highly pretreated adults and adolescents 12 years of age or older with virus resistant to multiple protease inhibitors.
Aptivus should only be used as part of an active combination antiretroviral regimen in patients with no other therapeutic options.
This indication is based on the results of two phase-III studies, performed in highly pretreated adult patients (median number of 12 prior antiretroviral agents) with virus resistant to protease inhibitors and of one phase-II study investigating pharmacokinetics, safety and efficacy of Aptivus in mostly treatment-experienced adolescent patients aged 12 to 18 years.
In deciding to initiate treatment with Aptivus, co-administered with low dose ritonavir, careful consideration should be given to the treatment history of the individual patient and the patterns of mutations associated with different agents. Genotypic or phenotypic testing (when available) and treatment history should guide the use of Aptivus. Initiation of treatment should take into account the combinations of mutations which may negatively impact the virological response to Aptivus, co-administered with low-dose ritonavir.
Tipranavir is a non-peptidic protease inhibitor (PI) of HIV. Protease inhibitors block the part of HIV called protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Nelfinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs.
Anti-HIV Agents
Agents used to treat AIDS and/or stop the spread of the HIV infection. These do not include drugs used to treat symptoms or opportunistic infections associated with AIDS. (See all compounds classified as Anti-HIV Agents.)
J05AE09
J - Antiinfectives for systemic use
J05 - Antivirals for systemic use
J05A - Direct acting antivirals
J05AE - Protease inhibitors
J05AE09 - Tipranavir
Absorption
Absorption is limited, although no absolute quantification of absorption is available.
Tipranavir is extensively bound to plasma proteins (>99.9%). It binds to both human serum albumin and a-1-acid glycoprotein. The mean fraction of tipranavir (dosed without ritonavir) unbound in plasma was similar in clinical samples from healthy volunteers and HIV-1 positive patients. Total plasma tipranavir concentrations for these samples ranged from 9 to 82 uM. The unbound fraction of tipranavir appeared to be independent of total drug concentration over this concentration range.
US Natl Inst Health; DailyMed. Current Medication Information for APTIVUS (tipranavir) capsule, liquid filled APTIVUS (tipranavir) solution (April 2012). Available from, as of November 25, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=08982e49-d2eb-4b25-b01a-1be52fd669ef
Administration of (14)C-tipranavir to subjects (n=8) that received Aptivus/ritonavir 500/200 mg dosed to steady-state demonstrated that most radioactivity (median 82.3%) was excreted in feces, while only a median of 4.4% of the radioactive dose administered was recovered in urine. In addition, most radioactivity (56%) was excreted between 24 and 96 hours after dosing. The effective mean elimination half-life of tipranavir/ritonavir in healthy volunteers (n=67) and HIV-1 infected adult patients (n=120) was approximately 4.8 and 6.0 hours, respectively, at steady state following a dose of 500/200 mg twice daily with a light meal.
US Natl Inst Health; DailyMed. Current Medication Information for APTIVUS (tipranavir) capsule, liquid filled APTIVUS (tipranavir) solution (April 2012). Available from, as of November 25, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=08982e49-d2eb-4b25-b01a-1be52fd669ef
The pharmacokinetic and metabolite profiles of the antiretroviral agent tipranavir (TPV), administered with ritonavir (RTV), in nine healthy male volunteers were characterized. Subjects received 500-mg TPV capsules with 200-mg RTV capsules twice daily for 6 days. They then received a single oral dose of 551 mg of TPV containing 90 uCi of [(14)C]TPV with 200 mg of RTV on day 7, followed by twice-daily doses of unlabeled 500-mg TPV with 200 mg of RTV for up to 20 days. Blood, urine, and feces were collected for mass balance and metabolite profiling. Metabolite profiling and identification was performed using a flow scintillation analyzer in conjunction with liquid chromatography-tandem mass spectrometry. The median recovery of radioactivity was 87.1%, with 82.3% of the total recovered radioactivity excreted in the feces and less than 5% recovered from urine. Most radioactivity was excreted within 24 to 96 hr after the dose of ((14)C)TPV. Radioactivity in blood was associated primarily with plasma rather than red blood cells. Unchanged TPV accounted for 98.4 to 99.7% of plasma radioactivity. Similarly, the most common form of radioactivity excreted in feces was unchanged TPV, accounting for a mean of 79.9% of fecal radioactivity. The most abundant metabolite in feces was a hydroxyl metabolite, H-1, which accounted for 4.9% of fecal radioactivity. TPV glucuronide metabolite H-3 was the most abundant of the drug-related components in urine, corresponding to 11% of urine radioactivity. In conclusion, after the coadministration of TPV and RTV, unchanged TPV represented the primary form of circulating and excreted TPV and the primary extraction route was via the feces.
PMID:17485497 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1913264 Chen L et al; Antimicrob Agents Chemother 51 (7): 2436-44 (2007)
The in vitro plasma protein binding of tipranavir was very high (> 99.9%) in all species including humans, with only a slight trend towards saturation over the concentration range of 10 to 100 um. Tipranavir with or without ritonavir co-administration, distributed primarily in the liver, small intestine, large intestine, kidney and lung. Tipranavir did not cross the blood-brain barrier and did not readily partitioning into red blood cells.
European Medicines Agency (EMA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Aptivus, Scientific Discussion p.6 (2005). Available from, as of November 26, 2012: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000631/WC500025932.pdf
Following intravenous dosing, tipranavir demonstrated low clearance ranging from 0.08 L/hr/kg in dogs to 1.15 l/h/kg in mice. The Vss ranged from 0.13 L/kg in dogs to 0.51 L/kg in rats. TPV was eliminated rapidly with a terminal t1/2 ranging from 0.93 hr in dogs to 5.43 hr in rats. Following oral dosing, tipranavir exhibited a mean Tmax ranging from 0.5 to 8 hr in all species. In all species a moderate or poor oral bioavailability of tipranavir was revealed, due to a lack of absorption and/or intestinal metabolism. Whereas the bioavailability in rats showed moderately levels of 28.0%, the bioavailability in dogs (6.5% and 7.7%) and also in mice (11%) and rabbits (9.9%) was minimal. Food had no significant effect on tipranavir oral bioavailability in dogs. Ritonavir co-administration studies were performed to investigate the benefit gained by the combination. However the use of different doses of ritonavir for oral and intravenous PK of tipranavir does not allow a clear comparison of tipranavir bioavailability with or without ritonavir. With ritonavir co-administration, following intravenous dosing, tipranavir demonstrated low to moderate clearance ranging from 0.0182 L/hr/kg in rats to 3.00 L/hr/kg in mice. In rats and dogs, co-administration of ritonavir resulted in a 4- to 5-fold decrease in clearance for tipranavir, which would be consistent with inhibition of drug-metabolising enzymes by ritonavir.
European Medicines Agency (EMA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Aptivus, Scientific Discussion p.5 (2005). Available from, as of November 26, 2012: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000631/WC500025932.pdf
Hepatic. In vitro metabolism studies with human liver microsomes indicated that CYP 3A4 is the predominant CYP enzyme involved in tipranavir metabolism.
Tipranavir (TPV) is the first nonpeptidic protease inhibitor used for the treatment of drug-resistant HIV infection. Clinically, TPV is coadministered with ritonavir (RTV) to boost blood concentrations and increase therapeutic efficacy. The mechanism of metabolism-mediated drug interactions associated with RTV-boosted TPV is not fully understood. In the current study, TPV metabolism was investigated in mice using a metabolomic approach. TPV and its metabolites were found in the feces of mice but not in the urine. Principal component analysis of the feces metabolome uncovered eight TPV metabolites, including three monohydroxylated, three desaturated, one dealkylated, and one dihydroxylated. In vitro study using human liver microsomes recapitulated five TPV metabolites, all of which were suppressed by RTV. CYP3A4 was identified as the primary enzyme contributing to the formation of four TPV metabolites (metabolites II, IV, V, and VI), including an unusual dealkylated product arising from carbon-carbon bond cleavage. Multiple cytochromes P450 (2C19, 2D6, and 3A4) contributed to the formation of a monohydroxylated metabolite (metabolite III). In vivo, RTV cotreatment significantly inhibited eight TPV metabolic pathways. In summary, metabolomic analysis revealed two known and six novel TPV metabolites in mice, all of which were suppressed by RTV. The current study provides solid evidence that the RTV-mediated boosting of TPV is due to the modulation of P450-dependent metabolism.
PMID:20103582 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2872945 Li F et al; Drug Metab Dispos 38 (5): 871-8 (2010)
The pharmacokinetic and metabolite profiles of the antiretroviral agent tipranavir (TPV), administered with ritonavir (RTV), in nine healthy male volunteers were characterized. Subjects received 500-mg TPV capsules with 200-mg RTV capsules twice daily for 6 days. They then received a single oral dose of 551 mg of TPV containing 90 uCi of [(14)C]TPV with 200 mg of RTV on day 7, followed by twice-daily doses of unlabeled 500-mg TPV with 200 mg of RTV for up to 20 days. ... The most abundant metabolite in feces was a hydroxyl metabolite, H-1, which accounted for 4.9% of fecal radioactivity. TPV glucuronide metabolite H-3 was the most abundant of the drug-related components in urine, corresponding to 11% of urine radioactivity. ...
PMID:17485497 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1913264 Chen L et al; Antimicrob Agents Chemother 51 (7): 2436-44 (2007)
In vitro metabolism studies indicated that CYP3A4 is the predominant CYP isoform involved in tipranavir metabolism in humans. CYP3A isozyme was also identified in rat as the predominant CYP isoform involved in tipranavir metabolism.
European Medicines Agency (EMA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Aptivus, Scientific Discussion p.7 (2005). Available from, as of November 26, 2012: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000631/WC500025932.pdf
Studies in rats and humans dosed by tipranavir co-administered with ritonavir were conducted to assess metabolites. The unchanged tipranavir was the predominant form in plasma (>85.7%). Unchanged tipranavir was also the major form excreted in feces and urine. Combined levels of excreted metabolites in feces and urine accounted for approximately 4.8% and 7.4% in male and female rats. Only small amounts of a glucuronide were observed in faeces.
European Medicines Agency (EMA), The European Agency for the Evaluation of Medicinal Products, European Public Assessment Report (EPAR) for Authorized Medicinal Products for Human Use; Aptivus, Scientific Discussion p.6 (2005). Available from, as of November 26, 2012: https://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000631/WC500025932.pdf
For more Metabolism/Metabolites (Complete) data for Tipranavir (6 total), please visit the HSDB record page.
5-6 hours
Tipranavir (TPV) is a non-peptidic HIV-1 protease inhibitor that inhibits the processing of the viral Gag and Gag-Pol polyproteins in HIV-1 infected cells, thus preventing formation of mature virions. Two mechanisms are suggested in regards to the potency of tipranavir: 1. Tipravanir may bind to the active site of the protease enzyme with fewer hydrogen bonds than peptidic protease inhibitors, which results in increased flexibility, allowing it to fit into the active site of the enzyme in viruses that have become resistance to other protease inhibitors. This also enables tipranavir to adjust to amino acid substitutions at the active site. 2. Tipranavir's strong hydrogen bonding interaction with the amide backbone of the protease active site Asp30 may lead to its activity against resistant viruses.
Tipranavir (TPV) is an HIV-1 protease inhibitor that inhibits the virus-specific processing of the viral Gag and Gag-Pol polyproteins in HIV-1 infected cells, thus preventing formation of mature virions.
US Natl Inst Health; DailyMed. Current Medication Information for APTIVUS (tipranavir) capsule, liquid filled APTIVUS (tipranavir) solution (April 2012). Available from, as of November 25, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=08982e49-d2eb-4b25-b01a-1be52fd669ef
Tipranavir inhibits the replication of laboratory strains of HIV-1 and clinical isolates in acute models of T-cell infection, with 50% effective concentrations (EC50) ranging from 0.03 to 0.07 uM (18-42 ng/mL). Tipranavir demonstrates antiviral activity in cell culture against a broad panel of HIV-1 group M non-clade B isolates (A, C, D, F, G, H, CRF01 AE, CRF02 AG, CRF12 BF). Group O and HIV-2 isolates have reduced susceptibility in cell culture to tipranavir with EC50 values ranging from 0.164 -1 uM and 0.233-0.522 uM, respectively. When used with other antiretroviral agents in cell culture, the combination of tipranavir was additive to antagonistic with other protease inhibitors (amprenavir, atazanavir, indinavir, lopinavir, nelfinavir, ritonavir, and saquinavir) and generally additive with the NNRTIs (delavirdine, efavirenz, and nevirapine) and the NRTIs (abacavir, didanosine, emtricitabine, lamivudine, stavudine, tenofovir, and zidovudine). Tipranavir was synergistic with the HIV-1 fusion inhibitor enfuvirtide. There was no antagonism of the cell culture combinations of tipranavir with either adefovir or ribavirin, used in the treatment of viral hepatitis.
US Natl Inst Health; DailyMed. Current Medication Information for APTIVUS (tipranavir) capsule, liquid filled APTIVUS (tipranavir) solution (April 2012). Available from, as of November 25, 2012: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=08982e49-d2eb-4b25-b01a-1be52fd669ef


