



API Suppliers

US DMFs Filed
0

CEP/COS Certifications

JDMFs Filed
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
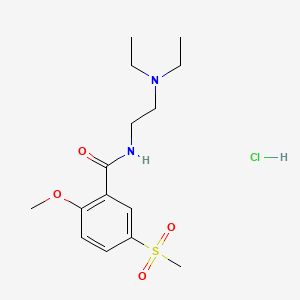

1. Equilium
2. Flo 1347
3. Flo-1347
4. Flo1347
5. Hydrochloride, Tiapride
6. Monohydrochloride, Tiapride
7. Tiapridal
8. Tiapride
9. Tiapride Monohydrochloride
10. Tiapridex
11. Tiaprizal
1. 51012-33-0
2. Tiapride Hcl
3. Tiapridal
4. Tiapride (hydrochloride)
5. Gramalil
6. Italprid
7. Luxoben
8. Sereprile
9. Tiapridex
10. Tiapridehydrochloride
11. Mls000069678
12. N-[2-(diethylamino)ethyl]-5-(methylsulfonyl)-o-anisamide Hydrochloride
13. Tiapride Hydrochloride [jan]
14. Benzamide, N-[2-(diethylamino)ethyl]-2-methoxy-5-(methylsulfonyl)-,monohydrochloride
15. Smr000058514
16. Dsstox_cid_25210
17. Dsstox_rid_80753
18. Dsstox_gsid_45210
19. N-(2-(diethylamino)ethyl)-2-methoxy-5-(methylsulfonyl)benzamide Hydrochloride
20. 25n106wedo
21. Sr-01000000232
22. Ncgc00015996-02
23. Cas-51012-33-0
24. N-(2-[diethylamino]ethyl)-5-(methylsulfonyl)-o-anisamide Hydrochloride
25. N-[2-(diethylamino)ethyl]-2-methoxy-5-(methylsulfonyl)benzamide Hydrochloride
26. Gramalil (tn)
27. Prestwick_580
28. Mfcd00133861
29. Benzamide, N-(2-(diethylamino)ethyl)-2-methoxy-5-(methylsulfonyl)-, Monohydrochloride
30. Opera_id_408
31. Tiapride Hydrochloride,(s)
32. Mls001076118
33. Schembl355315
34. Spectrum1503086
35. Tiapride Hydrochloride (jp17)
36. Chembl1256772
37. Dtxsid4045210
38. Chebi:32220
39. Hms1568l13
40. Hms1922e05
41. Pharmakon1600-01503086
42. Tiapride Hydrochloride [mi]
43. Bcp13379
44. Hy-b1196
45. Tox21_110276
46. Tox21_501124
47. Ccg-39299
48. Nsc758225
49. Akos015891372
50. Tiapride Hydrochloride [mart.]
51. Tox21_110276_1
52. Ab03720
53. Cs-4804
54. Hs-0005
55. Lp01124
56. Tiapride Hydrochloride [who-dd]
57. Ncgc00015996-10
58. Ncgc00094392-01
59. Ncgc00094392-02
60. Ncgc00094392-03
61. Ncgc00094392-04
62. Ncgc00094392-05
63. Ncgc00261809-01
64. Bt166255
65. Eu-0101124
66. Ft-0630638
67. S9509
68. T3600
69. Tiapride Hydrochloride [ep Monograph]
70. D01522
71. T 0410
72. T72548
73. Sr-01000000232-2
74. Sr-01000000232-6
75. Q27253983
76. N-[2-(diethylamino)ethyl]-2-methoxy-5-methylsulfonylbenzamide,hydrochloride
77. Benzamide, N-(2-(diethylamino)ethyl)-2-methoxy-5-(methylsulfonyl)-, Hydrochloride (1:1)
| Molecular Weight | 364.9 g/mol |
|---|---|
| Molecular Formula | C15H25ClN2O4S |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 5 |
| Rotatable Bond Count | 8 |
| Exact Mass | 364.1223562 g/mol |
| Monoisotopic Mass | 364.1223562 g/mol |
| Topological Polar Surface Area | 84.1 Ų |
| Heavy Atom Count | 23 |
| Formal Charge | 0 |
| Complexity | 443 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antipsychotic Agents
Agents that control agitated psychotic behavior, alleviate acute psychotic states, reduce psychotic symptoms, and exert a quieting effect. They are used in SCHIZOPHRENIA; senile dementia; transient psychosis following surgery; or MYOCARDIAL INFARCTION; etc. These drugs are often referred to as neuroleptics alluding to the tendency to produce neurological side effects, but not all antipsychotics are likely to produce such effects. Many of these drugs may also be effective against nausea, emesis, and pruritus. (See all compounds classified as Antipsychotic Agents.)
Dopamine Antagonists
Drugs that bind to but do not activate DOPAMINE RECEPTORS, thereby blocking the actions of dopamine or exogenous agonists. Many drugs used in the treatment of psychotic disorders (ANTIPSYCHOTIC AGENTS) are dopamine antagonists, although their therapeutic effects may be due to long-term adjustments of the brain rather than to the acute effects of blocking dopamine receptors. Dopamine antagonists have been used for several other clinical purposes including as ANTIEMETICS, in the treatment of Tourette syndrome, and for hiccup. Dopamine receptor blockade is associated with NEUROLEPTIC MALIGNANT SYNDROME. (See all compounds classified as Dopamine Antagonists.)


