



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. Lx1032
2. Xermelo
1. 1033805-22-9
2. Lx1606
3. Lx 1606
4. Xermelo
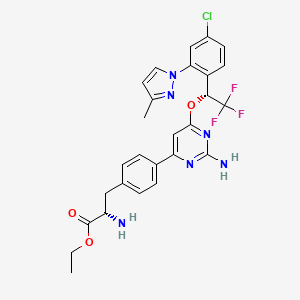
5. (s)-ethyl 2-amino-3-(4-(2-amino-6-((r)-1-(4-chloro-2-(3-methyl-1h-pyrazol-1-yl)phenyl)-2,2,2-trifluoroethoxy)pyrimidin-4-yl)phenyl)propanoate
6. Lx-1606
7. Telotristat Ethyl [usan]
8. Lx 1032
9. Lx1032
10. Lx-1032
11. Ethyl (2s)-2-amino-3-[4-[2-amino-6-[(1r)-1-[4-chloro-2-(3-methylpyrazol-1-yl)phenyl]-2,2,2-trifluoroethoxy]pyrimidin-4-yl]phenyl]propanoate
12. 8g388563m7
13. Telotristat Ethyl (usan)
14. Lx-1606 Hippurate
15. Telotristat Etiprate (lx 1606 Hippurate)
16. Telotristat-ethyl
17. Unii-8g388563m7
18. Xermelo (tn)
19. Schembl610588
20. Telotristat Ethyl [mi]
21. Gtpl9490
22. Chembl2105695
23. Ex-a322
24. Bdbm445704
25. Dtxsid001102271
26. Telotristat Ethyl [who-dd]
27. Amy38688
28. Hy-13055a
29. Lx1606lx1606
30. Mfcd18251583
31. Zinc43205655
32. Bcp9000866
33. Db12095
34. Us10683309, Example Lx1606
35. Ncgc00390241-01
36. Ncgc00390241-07
37. As-35061
38. D09974
39. J-524318
40. Q27270371
41. Ethyl 4-(2-amino-6-((1r)-1-(4-chloro-2-(3-methyl-1h-pyrazol-1-yl)phenyl)-2,2,2-trifluoroethoxy)pryrimidin-4-yl)-l-phenylalaninate
42. L-phenylalanine, 4-(2-amino-6-((1r)-1-(4-chloro-2-(3-methyl-1h-pyrazol-1-yl)phenyl)-2,2,2-trifluoroethoxy)-4-pryrimidinyl)-, Ethyl Ester
| Molecular Weight | 575.0 g/mol |
|---|---|
| Molecular Formula | C27H26ClF3N6O3 |
| XLogP3 | 5.1 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 10 |
| Exact Mass | 574.1707009 g/mol |
| Monoisotopic Mass | 574.1707009 g/mol |
| Topological Polar Surface Area | 131 Ų |
| Heavy Atom Count | 40 |
| Formal Charge | 0 |
| Complexity | 821 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 1 | |
|---|---|
| Drug Name | XERMELO |
| Active Ingredient | TELOTRISTAT ETIPRATE |
| Company | LEXICON PHARMS INC (Application Number: N208794. Patents: 7553840, 7709493, 7968559, 8193204, 8653094) |
As a serotonin synthesis inhibitor, telotristat etiprate, has the potential to reduce serotonin levels and address the key elements of carcinoid syndrome.
Xermelo is indicated for the treatment of carcinoid syndrome diarrhoea in combination with somatostatin analogue (SSA) therapy in adults inadequately controlled by SSA therapy.
Activity is mainly in the gastrointestinal tract, with minimal effects reported on the brain and cardiovascular system, accompanied by an excellent safety profile. In normal mice, telotristat etiprate (administered once daily for 4 days at doses of 15300 mg/kg/day) was found to reduce serotonin levels throughout the gastrointestinal tract. These reductions occurred in a dose dependent fashion with maximal effects observed with doses of telotristat etiprate 150 mg/kg. No significant change in brain serotonin or 5-hydroxyindoleacetic acid (5-HIAA, a serotonin metabolite) was observed. Similar findings were seen in Sprague-Dawley rats. Gastrointestinal motility studies were conducted in rats using the charcoal meal test. There was a significant dose-related delay in both gastrointestinal transit and gastric emptying, associated with a reduction in blood serotonin levels and proximal colon serotonin. A quantitative whole-body autoradiography study was conducted to assess the absorption, distribution and excretion of radioactivity in rats following a single oral dose of telotristat etiprate labeled with carbon 14. Rats were administered either 30 mg/kg or 100 mg/kg of the compound. The distribution of radioactivity was limited to tissues of the hepatic and renal system and the contents of the GI tract. There was no measurable radioactivity in the brain at any dose tested.
A16A
Absorption
Low systemic absorption. Tmax of 24 h, supporting the 8-hourly administration
Route of Elimination
Telotristat ethyl is mainly excreted in feces.
Clearance
Not available
Metabolized by the liver.
412 h
Telotristat etiprate is an ethyl ester prodrug which is hydrolyzed to its active moiety LP-778902 both in vivo and in vitro. Systemic exposure of telotristat etiprate is relatively low, as the hydrolysis to the active moiety is rapid. LP-778902 is a potent inhibitor of TPH with an in vivo IC50 of 0.028 M. While existing treatments for carcinoid syndrome work to reduce the release of serotonin outside tumor cells, telotristat etiprate works at the source to reduce serotonin production within the tumor cells. By specifically inhibiting serotonin production, telotristat etiprate seeks to control this important driver of carcinoid syndrome and, in turn, provide patients with more control over their disease.






