



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
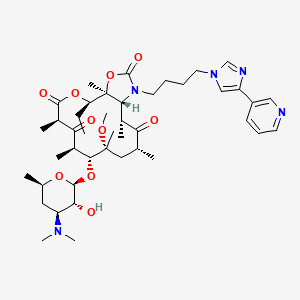

1. Hmr 3647
2. Hmr-3647
3. Hmr3647
4. Ketek
5. Ru 66647
6. Ru-66647
1. Ketek
2. 191114-48-4
3. Hmr 3647
4. Levviax
5. Hmr-3647
6. Hmr3647
7. Ru-66647
8. Ki8h7h19wl
9. Nsc-758940
10. Dsstox_cid_26455
11. Dsstox_rid_81629
12. Dsstox_gsid_46455
13. 2h-oxacyclotetradecino[4,3-d]oxazole-2,6,8,14(1h,7h,9h)-tetrone, 4-ethyloctahydro-11-methoxy-3a,7,9,11,13,15-hexamethyl-1-[4-[4-(3-pyridinyl)-1h-imidazol-1-yl]butyl]-10-[[3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranosyl]oxy]-, (3as,4r,7r,9r,10r,11r,13r,15r,15ar)-
14. Ru 66647
15. Ru66647
16. Cas-191114-48-4
17. Unii-ki8h7h19wl
18. Telithromycin [usan:inn:ban]
19. Hsdb 7359
20. Ncgc00164575-01
21. Telithromycin [mi]
22. Telithromycin [inn]
23. Telithromycin [jan]
24. Schembl5100
25. Chembl1136
26. Telithromycin [hsdb]
27. Telithromycin [usan]
28. Telithromycin [vandf]
29. Telithromycin [mart.]
30. Telithromycin [who-dd]
31. Dtxsid3046455
32. Telithromycin [ema Epar]
33. Chebi:29688
34. Telithromycin [orange Book]
35. Hy-a0062
36. Zinc9574770
37. Tox21_111454
38. Tox21_112203
39. Bdbm50378137
40. Mfcd00943561
41. Db00976
42. Nsc 758940
43. Hmr-3647;ru-66647
44. 11,12-dideoxy-3-des(2,6-dideoxy-3-c,3-o-dimethyl-alpha-l-altropyranosyloxy)-6-o-methyl-3-oxo-12,11-(oxycarbonylimino)-n11-[4-[4-(3-pyridyl)imidazol-1-yl]butyl]erythromycin A
45. Q2736135
46. (1r,2r,4r,6r,7r,8r,10r,13r,14s)-7-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyl-tetrahydropyran-2-yl]oxy-13-ethyl-6-methoxy-2,4,6,8,10,14-hexamethyl-17-[4-[4-(3-pyridyl)imidazol-1-yl]butyl]-12,15-dioxa-17-azabicyclo[12.3.0]heptadecane-3,9,11,16-tetrone
47. (1s,2r,5r,7r,8r,9r,11r,13r,14r)-8-[(2s,3r,4s,6r)-4-(dimethylamino)-3-hydroxy-6-methyloxan-2-yl]oxy-2-ethyl-9-methoxy-1,5,7,9,11,13-hexamethyl-15-[4-(4-pyridin-3-ylimidazol-1-yl)butyl]-3,17-dioxa-15-azabicyclo[12.3.0]heptadecane-4,6,12,16-tetrone
48. (3as,4r,7r,9r,10r,11r,13r,15r,15ar)-4-ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-1-(4-(4-(pyridin-3-yl)-1h-imidazol-1-yl)butyl)-10-((3,4,6-trideoxy-3-(dimethylamino)-b-d-xylo-hexopyranosyl)oxy)octahydro-2h-oxacyclotetradecino(4,3-d)oxazole-2,6,8,14(1h,7h,9h)-tetrone
49. (3as,4r,7r,9r,10r,11r,13r,15r,15ar)-4-ethyl-11-methoxy-3a,7,9,11,13,15-hexamethyl-2,6,8,14-tetraoxo-1-{4-[4-(pyridin-3-yl)-1h-imidazol-1-yl]butyl}tetradecahydro-2h-oxacyclotetradecino[4,3-d][1,3]oxazol-10-yl 3,4,6-trideoxy-3-(dimethylamino)-beta-d-xylo-hexopyranoside
50. Erythromycin, 3-de((2,6-dideoxy-3-c-methyl-3-o-methyl-alpha-l-ribo-hexopyranosyl)oxy)-11,12-dideoxy-6-o-methyl-3-oxo-12,11-(oxycarbonyl((4-(4-(3-pyridinyl)-1h-imidazol-1-yl)butyl)imino))-
51. Erythromycin, 3-de[(2,6-dideoxy-3-c-methyl-3-o-methyl-.alpha.-l-ribo-hexopyranosyl)oxy]-11,12-dideoxy-6-o-methyl-3-oxo-12,11-[oxycarbonyl[[4-[4-(3-pyridinyl)-1h-imidazol-1-yl]butyl]imino]]-
52. Erythromycin,3-de((2,6-dideoxy-3-c-methyl-3-o-methyl-.alpha.-l-ribo-hexopyranosyl)oxy)-11,12-dideoxy-6-o-methyl-3-oxo-12,11-(oxycarbonyl((4-(4-(3-pyridinyl)-1h-imidazol-1-yl)butyl)imino)-
| Molecular Weight | 812.0 g/mol |
|---|---|
| Molecular Formula | C43H65N5O10 |
| XLogP3 | 4.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 11 |
| Exact Mass | 811.47314329 g/mol |
| Monoisotopic Mass | 811.47314329 g/mol |
| Topological Polar Surface Area | 172 Ų |
| Heavy Atom Count | 58 |
| Formal Charge | 0 |
| Complexity | 1440 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 13 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Ketek |
| PubMed Health | Telithromycin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | KETEK tablets contain telithromycin, a semisynthetic antibacterial in the ketolide class for oral administration. Chemically, telithromycin is designated as Erythromycin, 3-de[(2,6-dideoxy-3-C-methyl-3-O-methyl--L-ribo-hexopyranosyl)oxy]-11,12-di... |
| Active Ingredient | Telithromycin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 400mg; 300mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
| 2 of 2 | |
|---|---|
| Drug Name | Ketek |
| PubMed Health | Telithromycin (By mouth) |
| Drug Classes | Antibiotic |
| Drug Label | KETEK tablets contain telithromycin, a semisynthetic antibacterial in the ketolide class for oral administration. Chemically, telithromycin is designated as Erythromycin, 3-de[(2,6-dideoxy-3-C-methyl-3-O-methyl--L-ribo-hexopyranosyl)oxy]-11,12-di... |
| Active Ingredient | Telithromycin |
| Dosage Form | Tablet |
| Route | Oral |
| Strength | 400mg; 300mg |
| Market Status | Prescription |
| Company | Sanofi Aventis Us |
Antibacterial
O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 1627
Telithromycin in indicated in the treatment of acute bacterial exacerbation of chronic bronchitis due to Streptococcus pneumoniae, Haemophilus influenzae, or Moraxella catarrhalis. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 2726
Telithromycin is indicated for the treatment of group A-beta Hemolytic Streptococcus as an alternative when beta-lactam antibiotics are not appropriate. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 2726
Telithromycin is indicated in the treatment of community-acquired pneumonia due to Streptococcus pneumoniae (including multi-drug resistant isolates (MDRSP)), Haemophilus influenzae, Chlamydophila pneumoniae, Mycoplasma pneumoniae, or Moraxella catarrhalis. /NOT included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 2726
Telithromycin is indicated in the treatment of acute bacterial sinusitis due to Steptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, or Staphylococcus aureus. /Included in US product labeling/
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 2726
Pseudomembranous colitis has been reported with nearly all antibacterial agents, including telithromycin, and may range in severity from mild to life-threatening. Therefore, it is important to consider this diagnosis in patients who present with diarrhea subsequent to the administration of any antibacterial agents.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 711
FDA Pregnancy Risk Category: C /RISK CANNOT BE RULED OUT. Adequate, well controlled human studies are lacking, and animal studies have shown risk to the fetus or are lacking as well. There is a chance of fetal harm if the drug is given during pregnancy; but the potential benefits may outweigh the potential risk./
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 2727
Treatment with anti-infectives, including telithromycin, may permit overgrowth of clostridia. Consider Clostridium difficile-associated diarrhea and colitis (antibiotic-associated pseudomembranous colitis) if diarrhea develops and manage accordingly. Some mild cases of C. difficile-asssociated diarrhea and colitis may respond to discontinuance alone. Manage moderate to severe cases with fluid, electrolyte, and protein supplementation; appropriate anti-infective therapy (e.g., oral metronidazole or vancomycin) recommended if colitis is severe
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 231
To reduce development of drug-resistant bacteria and maintain effectiveness of telithromycin and other antibacterials, use only for treatment of infections proven or strongly suspected to be caused by susceptible bacteria. When selecting or modifying anti-infective therapy, use results of culture and in vitro susceptibility testing. In the absence of such data, consider local epidemiology and susceptibility patterns when selecting anti-infectives for empiric therapy.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 232
For more Drug Warnings (Complete) data for TELITHROMYCIN (14 total), please visit the HSDB record page.
For the treatment of Pneumococcal infection, acute sinusitis, acute bacterial tonsillitis, acute bronchitis and bronchiolitis, lower respiratory tract infection and lobar (pneumococcal) pneumonia.
FDA Label
When prescribing Ketek, consideration should be given to official guidance on the appropriate use ofantibacterial agents and the local prevalence of resistance.
Ketek is indicated for the treatment of the following infections:
* In patients of 18 years and older:
- community-acquired pneumonia, mild or moderate.
- when treating infections caused by known or suspected beta-lactam- and / or macrolide-resistant strains (according to history of patients or national and / or regional resistance data) covered by the antibacterial spectrum of telithromycin:
- acute exacerbation of chronic bronchitis;
- acute sinusitis;
* In patients of 12 years and older:
- tonsillitis / pharyngitis caused by Streptococcus pyogenes, as an alternative when beta-lactam antibiotics are not appropriate in countries / regions with a significant prevalence of macrolide-resistant S. pyogenes, when mediated by ermTR or mefA.
When prescribing Levviax consideration should be given to official guidance on the appropriate use of antibacterial agents and the local prevalence of resistance (see also sections 4. 4 and 5. 1).
Levviax is indicated for the treatment of the following infections:
In patients of 18 years and older:
-Community-acquired pneumonia, mild or moderate (see section 4. 4).
- When treating infections caused by known or suspected beta-lactam and/or macrolide resistant strains (according to history of patients or national and/or regional resistance data) covered by the antibacterial spectrum of telithromycin (see sections 4. 4 and 5. 1):
- Acute exacerbation of chronic bronchitis,
- Acute sinusitis
In patients of 12 years and older:
- Tonsillitis/pharyngitis caused by Streptococcus pyogenes, as an alternative when beta lactam antibiotics are not appropriate in countries/regions with a significant prevalence of macrolide resistant S. pyogenes, when mediated by ermTR or mefA (see sections 4. 4 and 5. 1).
Telithromycin is a ketolide antibiotic which has an antimicrobial spectrum similar or slightly broader than that of penicillin. It is often used as an alternative in patients who have an allergy to penicillins. For respiratory tract infections, it has better coverage of atypical organisms, including mycoplasma. Telithromycin prevents bacterial growth by binding to bacterial 50S ribosomal subunits and interfering with bacterial peptide translocation and elongation.
Anti-Bacterial Agents
Substances that inhibit the growth or reproduction of BACTERIA. (See all compounds classified as Anti-Bacterial Agents.)
J01FA15
J01FA15
J - Antiinfectives for systemic use
J01 - Antibacterials for systemic use
J01F - Macrolides, lincosamides and streptogramins
J01FA - Macrolides
J01FA15 - Telithromycin
Absorption
Absolute bioavailability is approximately 57%. Maximal concentrations are reached 0.5 - 4 hours following oral administration. Food intake does not affected absorption.
Route of Elimination
The systemically available telithromycin is eliminated by multiple pathways as follows: 7% of the dose is excreted unchanged in feces by biliary and/or intestinal secretion; 13% of the dose is excreted unchanged in urine by renal excretion; and 37% of the dose is metabolized by the liver.
Volume of Distribution
2.9 L/kg
Telithromycin concentration in white blood cells exceeds the concentration in plasma and is eliminated more slowly from white blood cells than from plasma. Mean white blood cell concentrations of telithromycin peaked at 72.1 ug/mL at 6 hours, and remained at 14.1 ug/mL 24 hours after 5 days of repeated dosing of 600 mg once daily. After 10 days, repeated dosing of 600 mg once daily, white blood cell concentrations remained at 8.9 ug/mL 48 hours after the last dose.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 710
Volume of distribution - 2.9 L/kg following an intervenous infusion. Widely distributed throughout the body. Telithromycin is distributed into breast milk of rats.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 2726
Rapidly absorbed, absolute bioavailability is 57% in both young and elderly patients. The rate and extent of absorption are unaffected by food. The AUC following a single dose is 8.25 ug h/mL and 12.5 ug h/mL following multiple dosing.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 2726
The systemically available telithromycin is eliminated by multiple pathways as follows: 7% of the dose is excreted unchanged in feces by biliary and/or intestinal secretion; 13% of the dose is excreted unchanged in urine by renal excretion; and 37% of the dose is metabolized by the liver.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 710
For more Absorption, Distribution and Excretion (Complete) data for TELITHROMYCIN (11 total), please visit the HSDB record page.
Hepatic - estimated 50% metabolized by CYP3A4 and 50% metabolized independent of cytochrome P450
About 70% (33% presystemic and 37% systemic) of an oral dose is metabolized about equally by cytochrome P450 (CYP) 3A4 and non-CYP3A4 isoenzymes to 4 major metabolites. The systemically available telithromycin is eliminated in the feces as unchanged drug (7%), in urine as unchanged drug (13%), and 37% is metabolized in the liver.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 233
Biotransformation: Hepatic; 37% of the dose is metabolized by the liver. Metabolism accounts for approximately 70% of the dose. The main metabolite represented 12.6% of the AUC while three other metabolites quantified represented 3% or less of the AUC of telithromycin.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 2726
In total, metabolism accounts for approximately 70% of the dose. In plasma, the main circulating compound after administration of an 800-mg radiolabeled dose was parent compound, representing 56.7% of the total radioactivity. The main metabolite represented 12.6% of the AUC of telithromycin. Three other plasma metabolites were quantified, each representing 3% or less of the AUC of telithromycin. It is estimated that approximately 50% of its metabolism is mediated by CYP 450 3A4 and the remaining 50% is CYP 450-independent.
Physicians Desk Reference. 59th ed. Thomson PDR. Montvale, NJ 2005., p. 710
Main elimination half-life is 2-3 hours; terminal elimination half-life is 10 hours
Elimination: 10 hours following oral dosing.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 2726
Telithromycin acts by binding to domains II and V of 23S rRNA of the 50S ribosomal subunit. By binding at domain II, telithromycin retains activity against gram-positive cocci (e.g. Streptococcus pneumoniae) in the presence of resistance mediated by methylases (erm genes) that alter the binding site at domain V. Telithromycin may also inhibit the assembly of nascent ribosomal units. Compared to erythromycin A, telithromycin binds to the 23S rRNA with 10 times greater affinity in erythromycin-susceptible organisms and 25 times greater affinity in macrolide-resistant strains. This increased binding affinity may be conferred by the C11-12 carbamate side chain of telithromycin. The side chain appears to maintain binding at domain II in the presence of resistance mediated by alterations in domain V.
Telithromycin may be bacteriostatic or bactericidal in action. Like conventional macrolides, telithromycin inhibits protein synthesis in susceptible organisms by binding to the 50S ribosomal subunit. Telithromycin binds to domains II and V of the 23S rRNA of the 50S subunit and has a higher affinity for these ribosomal targets than conventional macrolides, apparently because of additional interactions and increased binding at domain II. This allows telithromycin to retain activity against some gram-positive cocci (e.g., some strains ofS. pneumoniae) that have methylase-mediated resistance (erm genes) that alter the domain V binding site. In addition to inhibiting protein synthesis, telithromycin may inhibit assembly of nascent ribosomal units.
McEvoy, G.K. (ed.). American Hospital Formulary Service- Drug Information 2005. Bethesda, MD: American Society of Health-System Pharmacists, Inc. 2005 (Plus Supplements)., p. 233
Telithromycin blocks protein synthesis by binding to two sites on the 50S ribosomal subunit: domain II and V of the 23S rRNA. The greater affinity in binding strength can be attributed to the C11-C12 carbamate side chain.
Thomson.Micromedex. Drug Information for the Health Care Professional. 25th ed. Volume 1. Plus Updates. Content Reviewed by the United States Pharmacopeial Convention, Inc. Greenwood Village, CO. 2005., p. 2726






