



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
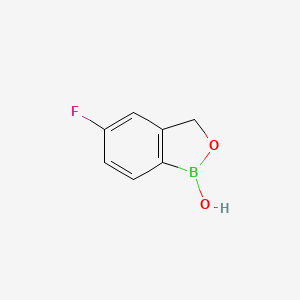

1. 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole
2. An 2690
3. An-2690
4. An2690
5. Kerydin
1. 174671-46-6
2. An-2690
3. 5-fluorobenzo[c][1,2]oxaborol-1(3h)-ol
4. Kerydin
5. An2690
6. 2,1-benzoxaborole, 5-fluoro-1,3-dihydro-1-hydroxy-
7. An 2690
8. 5-fluoro-1-hydroxy-3h-2,1-benzoxaborole
9. Tavaborole [usan]
10. 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole
11. 5-fluoro-1,3-dihydro-2,1-benzoxaborol-1-ol
12. K124a4euq3
13. Chebi:77942
14. Tavaborole (usan)
15. Mfcd10699483
16. 5-fluoro-2,1-benzoxaborol-1(3h)-ol
17. 5-fluoro-3h-benzo[c][1,2)oxaborol-1-ol
18. 5-fluoro-3h-benzo[c][1,2]oxaborol-1-ol
19. Tavaborole [usan:inn]
20. Unii-k124a4euq3
21. P-fluorbenzoxaborole
22. Kerydin (tn)
23. Sch-900340
24. Tavaborole [mi]
25. Tavaborole [inn]
26. An-2690(tavaborole)
27. Tavaborole [vandf]
28. Tavaborole (an-2690)
29. Tavaborole [who-dd]
30. Schembl500016
31. Chembl443052
32. Tavaborole [orange Book]
33. Hsdb 8342
34. Dtxsid00169888
35. Bcp08730
36. Ex-a1086
37. Bdbm50370987
38. S4996
39. Akos006303927
40. Zinc169990691
41. Ccg-266215
42. Cs-1058
43. Db09041
44. Ds-8392
45. Mb08883
46. 5-fluoro-1-hydroxy-2,1-benzoxaborolane
47. Ncgc00264110-01
48. Ncgc00264110-02
49. Ac-30887
50. Hy-10980
51. Sy038332
52. Db-100333
53. Ft-0697827
54. T3775
55. A14999
56. D10169
57. F11396
58. 1,3-dihydro-5-fluoro-1-hydroxy-2,1-benzoxaborole
59. Q21011226
60. 5-fluoro-1-hydroxyl-1,3-dihydrobenzo[c][1,2]oxaborole
61. Z1739256284
| Molecular Weight | 151.93 g/mol |
|---|---|
| Molecular Formula | C7H6BFO2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 0 |
| Exact Mass | 152.0444878 g/mol |
| Monoisotopic Mass | 152.0444878 g/mol |
| Topological Polar Surface Area | 29.5 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 155 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Antifungal Agents
National Library of Medicine's Medical Subject Headings. Tavaborole. Online file (MeSH, 2016). Available from, as of June 24, 2016: https://www.nlm.nih.gov/mesh/2016/mesh_browser/MBrowser.html
/CLINICAL TRIALS/ ClinicalTrials.gov is a registry and results database of publicly and privately supported clinical studies of human participants conducted around the world. The Web site is maintained by the National Library of Medicine (NLM) and the National Institutes of Health (NIH). Each ClinicalTrials.gov record presents summary information about a study protocol and includes the following: Disease or condition; Intervention (for example, the medical product, behavior, or procedure being studied); Title, description, and design of the study; Requirements for participation (eligibility criteria); Locations where the study is being conducted; Contact information for the study locations; and Links to relevant information on other health Web sites, such as NLM's MedlinePlus for patient health information and PubMed for citations and abstracts for scholarly articles in the field of medicine. Tavaborole is included in the database.
NIH/NLM; ClinicalTrials.Gov. Available from, as of July 6, 2016: https://clinicaltrials.gov/search/intervention=AN2690+OR+AN+2690%20OR%20Tavaborole
Kerydin (tavaborole) topical solution, 5% is an oxaborole antifungal indicated for the treatment of onychomycosis of the toenails due to Trichophyton rubrum or Trichophyton mentagrophytes. /Included in US product label/
NIH; DailyMed. Current Medication Information for Kerydin (Tavaborole) Solution (Updated: January 2015). Available from, as of July 5, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6ba6f49-0055-4285-9e04-67001d4554fd
Adverse effects reported in at least 1% of adults treated with tavaborole 5% topical solution and more frequently than with topical vehicle solution include application site exfoliation, ingrown toenail, application site erythema, and application site dermatitis.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016
Tavaborole 5% topical solution may cause skin irritation; there is no evidence to date that the solution causes contact sensitization.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016
It is not known whether tavaborole is excreted in human milk following topical application of Kerydin. Because many drugs are excreted in human milk, caution should be exercised when Kerydin is administered to a nursing woman.
NIH; DailyMed. Current Medication Information for Kerydin (Tavaborole) Solution (Updated: January 2015). Available from, as of July 5, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6ba6f49-0055-4285-9e04-67001d4554fd
There are no adequate and well-controlled studies with Kerydin in pregnant women. Kerydin should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
NIH; DailyMed. Current Medication Information for Kerydin (Tavaborole) Solution (Updated: January 2015). Available from, as of July 5, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6ba6f49-0055-4285-9e04-67001d4554fd
For more Drug Warnings (Complete) data for Tavaborole (9 total), please visit the HSDB record page.
Indicated for the treatment of onychomycosis (a fungal infection) of the toenails due to Trichophyton rubrum or Trichophyton mentagrophytes.
FDA Label
After a single dose, the mean ( standard deviation) peak concentration (Cmax) of tavaborole was 3.54 2.26 ng/mL (n=21 with measurable concentrations, range 0.618-10.2 ng/mL, LLOQ=0.5 ng/mL), and the mean AUClast was 44.4 25.5 ng*hr/mL (n=21). After 2 weeks of daily dosing, the mean Cmax was 5.17 3.47 ng/mL (n=24, range 1.51-12.8 ng/mL), and the mean AUC was 75.8 44.5 ng*hr/mL.
Antifungal Agents
Substances that destroy fungi by suppressing their ability to grow or reproduce. They differ from FUNGICIDES, INDUSTRIAL because they defend against fungi present in human or animal tissues. (See all compounds classified as Antifungal Agents.)
D - Dermatologicals
D01 - Antifungals for dermatological use
D01A - Antifungals for topical use
D01AE - Other antifungals for topical use
D01AE24 - Tavaborole
Absorption
7.5%. Subungual onychomycosis is difficult to treat due to the poorly perfused location of the infection in the nailbed. To be effective, a topical treatment must penetrate the nail plate and reach the site of infection at a concentration sufficient to exert anti-fungal activity. Tavaborole was shown to produce anti-fungal effects after 5 days of topical administration.
Route of Elimination
Primarily renal.
The pharmacokinetics of tavaborole was investigated in 24 subjects with distal subungual onychomycosis involving at least 4 toenails (including at least 1 great toenail) following a single dose and a 2-week daily topical application of 200 uL of a 5% solution of tavaborole to all ten toenails and 2 mm of skin surrounding each toenail. Steady state was achieved after 14 days of dosing. After a single dose, the mean (+ or - standard deviation) peak concentration (Cmax) of tavaborole was 3.54 + or - 2.26 ng/mL (n=21 with measurable concentrations, range 0.618-10.2 ng/mL, LLOQ=0.5 ng/mL), and the mean AUClast was 44.4 + or - 25.5 ng*hr/mL (n=21). After 2 weeks of daily dosing, the mean Cmax was 5.17 + or - 3.47 ng/mL (n=24, range 1.51-12.8 ng/mL), and the mean AUCt was 75.8 + or - 44.5 ng*hr/mL.
NIH; DailyMed. Current Medication Information for Kerydin (Tavaborole) Solution (Updated: January 2015). Available from, as of July 5, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6ba6f49-0055-4285-9e04-67001d4554fd
Renal excretion is the major route of elimination. In a clinical pharmacology trial of six healthy adult male volunteers who received a single topical application of 5% (14)C-tavaborole solution, tavaborole conjugates and metabolites were shown to be excreted primarily in the urine.
NIH; DailyMed. Current Medication Information for Kerydin (Tavaborole) Solution (Updated: January 2015). Available from, as of July 5, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6ba6f49-0055-4285-9e04-67001d4554fd
/MILK/ It is not known whether tavaborole is excreted in human milk following topical application of Kerydin.
NIH; DailyMed. Current Medication Information for Kerydin (Tavaborole) Solution (Updated: January 2015). Available from, as of July 5, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6ba6f49-0055-4285-9e04-67001d4554fd
Onychomycosis is a common infection of the toenails that causes nail thickening and discoloration. The physical appearance of the infected nail can diminish self-image and negatively impact quality of life. Patients may use nail polish to mask the appearance of infected nails. /The purpose of this study was/ to evaluate the in vitro nail penetration properties of tavaborole topical solution, 5%, through nail polish using ex vivo, non-diseased human fingernails. In study 1, tavaborole penetration was evaluated over 20 days of dosing using the Franz finite dose technique and modified Franz diffusion cells. Nails received either 1 coat of over-the-counter (OTC) typical polish or were left unpolished (controls). In study 2, tavaborole penetration was measured over 14 days of dosing using the finite dose technique and vertical diffusion cells. Nails were polished with either 4 coats or 1 coat of salon typical polish or with 2 coats or 1 coat of OTC typical polish, or they were left unpolished. In study 1, the mean + or - standard deviation (SD) cumulative tavaborole penetration at day 21 was numerically higher, though not statistically significant, through polished nails (3,526 + or - 1,433 ug/sq cm)vs unpolished nails (2,661 + or - 1,319 ug/sq cm).In study 2, the mean cumulative tavaborole penetration was also numerically higher (statistical significance not assessed) through all nails that received polish vs unpolished nails. At day 15, mean + or - SD cumulative tavaborole nail penetration was 1,179 + or - 554 ug/sq cm through 4 coats of salon typical polish, 1,227 + or - 974 ug/sq cm through 1 coat of salon typical polish, 1,493 + or - 1,322 ug/sq cm through 2 coats of OTC typical polish, 1,428 + or - 841 ug/sq cm through 1 coat of OTC typical polish, and 566 + or - 318 ug/sq cm through unpolished nails. Results from these in vitro studies demonstrated that tavaborole penetrated through human nails with up to 4 layers of nail polish.
PMID:26151782 Vlahovic T et al; J Drugs Dermatol 14 (7): 675-8 (2015)
Tavaborole undergoes extensive metabolism. Metabolite profiling revealed trace levels of a sulfated-conjugate and a benzoic acid metabolite, consistent with the known biotransformation of tavaborole.
Tavaborole undergoes extensive metabolism. ... In a clinical pharmacology trial of six healthy adult male volunteers who received a single topical application of 5% (14)C-tavaborole solution, tavaborole conjugates and metabolites were shown to be excreted primarily in the urine.
NIH; DailyMed. Current Medication Information for Kerydin (Tavaborole) Solution (Updated: January 2015). Available from, as of July 5, 2016: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f6ba6f49-0055-4285-9e04-67001d4554fd
28.5 hr
Tavaborole exerts its antifungal activity by blocking cellular protein synthesis through the formation of an adduct with cytoplasmic leucyl-aminoacyl transfer RNA (tRNA) synthetase.
The broad-spectrum benzoxaborole antifungal AN2690 /tavaborole/ blocks protein synthesis by inhibiting leucyl-tRNA synthetase (LeuRS) via a novel oxaborole tRNA trapping mechanism in the editing site. Herein, one set of resistance mutations is at Asp487 outside the LeuRS hydrolytic editing pocket, in a region of unknown function. It is located within a eukaryote/archaea specific insert I4, which forms part of a cap over a benzoxaborole-AMP that is bound in the LeuRS CP1 domain editing active site. Mutational and biochemical analysis at Asp487 identified a salt bridge between Asp487 and Arg316 in the hinge region of the I4 cap of yeast LeuRS that is critical for tRNA deacylation. We hypothesize that this electrostatic interaction stabilizes the cap during binding of the editing substrate for hydrolysis.
PMID:21856301 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3225056 Sarkar J et al; FEBS Lett 585 (19): 2986-91 (2011)
The mechanism of action of tavaborole against susceptible fungi involves inhibition of fungal protein synthesis by inhibition of an aminoacyl-transfer ribonucleic acid (tRNA) synthetase (AARS). Tavaborole inhibits the editing domain of leucyl-tRNA synthetase and exhibits good relative selectivity for this fungal enzyme.
American Society of Health-System Pharmacists 2016; Drug Information 2016. Bethesda, MD. 2016
Leucyl-tRNA synthetase (LeuRS) specifically links leucine to the 3' end of tRNA(leu) isoacceptors. The overall accuracy of the two-step aminoacylation reaction is enhanced by an editing domain that hydrolyzes mischarged tRNAs, notably ile-tRNA(leu). We present crystal structures of the editing domain from two eukaryotic cytosolic LeuRS: human and fungal pathogen Candida albicans. In comparison with previous structures of the editing domain from bacterial and archeal kingdoms, these structures show that the LeuRS editing domain has a conserved structural core containing the active site for hydrolysis, with distinct bacterial, archeal, or eukaryotic specific peripheral insertions. It was recently shown that the benzoxaborole antifungal compound AN2690 (5-fluoro-1,3-dihydro-1-hydroxy-1,2-benzoxaborole) inhibits LeuRS by forming a covalent adduct with the 3' adenosine of tRNA(leu) at the editing site, thus locking the enzyme in an inactive conformation. To provide a structural basis for enhancing the specificity of these benzoxaborole antifungals, we determined the structure at 2.2 A resolution of the C. albicans editing domain in complex with a related compound, AN3018 (6-(ethylamino)-5-fluorobenzo[c][1,2]oxaborol-1(3H)-ol), using AMP as a surrogate for the 3' adenosine of tRNA(leu). The interactions between the AN3018-AMP adduct and C. albicans LeuRS are similar to those previously observed for bacterial LeuRS with the AN2690 adduct, with an additional hydrogen bond to the extra ethylamine group. However, compared to bacteria, eukaryotic cytosolic LeuRS editing domains contain an extra helix that closes over the active site, largely burying the adduct and providing additional direct and water-mediated contacts. Small differences between the human domain and the fungal domain could be exploited to enhance fungal specificity.
PMID:19426743 Seiradake E et al; J Mol Biol 390 (2): 196-207 (2009)
Aminoacyl-transfer RNA (tRNA) synthetases, which catalyze the attachment of the correct amino acid to its corresponding tRNA during translation of the genetic code, are proven antimicrobial drug targets. We show that the broad-spectrum antifungal 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole (AN2690), in development for the treatment of onychomycosis, inhibits yeast cytoplasmic leucyl-tRNA synthetase by formation of a stable tRNA(Leu)-AN2690 adduct in the editing site of the enzyme. Adduct formation is mediated through the boron atom of AN2690 and the 2'- and 3'-oxygen atoms of tRNA's3'-terminal adenosine. The trapping of enzyme-bound tRNA(Leu) in the editing site prevents catalytic turnover, thus inhibiting synthesis of leucyl-tRNA(Leu) and consequentially blocking protein synthesis. This result establishes the editing site as a bona fide target for aminoacyl-tRNA synthetase inhibitors.
PMID:17588934 Rock FL et al; Science 316 (5832): 1759-61 (2007)
A new class of antimicrobial benzoxaborole compounds was identified as a potent inhibitor of leucyl-tRNA synthetase (LeuRS) and therefore of protein synthesis. In a novel mechanism, AN2690 (5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole) blocks fungal cytoplasmic LeuRS by covalently trapping tRNA(Leu) in the editing site of the enzyme's CP1 domain. However, some resistant mutation sites are located outside of the CP1 hydrolytic editing active site. Thus, their mode of action that undermines drug inhibition was not understood. A combination of X-ray crystallography, molecular dynamics, metadynamics, biochemical experiments, and mutational analysis of a distal benzoxaborole-resistant mutant uncovered a eukaryote-specific tyrosine "switch" that is critical to tRNA-dependent post-transfer editing. The tyrosine "switch" has three states that shift between interactions with a lysine and the 3'-hydroxyl of the tRNA terminus, to inhibit or promote post-transfer editing. The oxaborole's mechanism of action capitalizes upon one of these editing active site states. This tunable editing mechanism in eukaryotic and archaeal LeuRSs is proposed to facilitate precise quality control of aminoacylation fidelity. These mechanistic distinctions could also be capitalized upon for development of the benzoxaboroles as a broad spectrum antibacterial.
PMID:26172575 Zhao H et al; ACS Chem Biol 10 (10): 2277-85 (2015)


