



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)

Europe

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
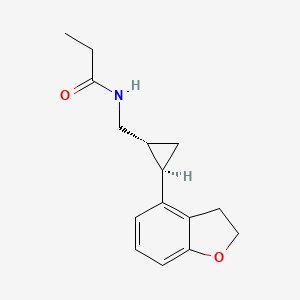

1. Bms 214778
2. Bms-214778
3. Bms214778
4. N-((2-(2,3-dihydro-4-benzofuranyl)cyclopropyl)methyl)propanamide
1. 609799-22-6
2. Hetlioz
3. Vec-162
4. Bms-214778
5. Vec 162
6. Bms 214778
7. Shs4pu80d9
8. Chebi:79042
9. Bms214778
10. Bms-214,778
11. N-(((1r,2r)-2-(2,3-dihydrobenzofuran-4-yl)cyclopropyl)methyl)propionamide
12. N-[[(1r,2r)-2-(2,3-dihydro-1-benzofuran-4-yl)cyclopropyl]methyl]propanamide
13. N-{[(1r,2r)-2-(2,3-dihydro-1-benzofuran-4-yl)cyclopropyl]methyl}propanamide
14. N-(((1r,2r)-2-(2,3-dihydro-1-benzofuran-4-yl)cyclopropyl)methyl)propanamide
15. Propanamide, N-(((1r,2r)-2-(2,3-dihydro-4-benzofuranyl)cyclopropyl)methyl)-
16. Tasimelteon [usan]
17. Tasimelteon [usan:inn]
18. Unii-shs4pu80d9
19. Tasimelteonum
20. N-([(1r,2r)-2-(2,3-dihydro-1-benzofuran-4-yl)cyclopropyl]methyl)propanamide
21. Hetlioz (tn)
22. Hetlioz Lq
23. Tasimelteon [mi]
24. Tasimelteon [inn]
25. Tasimelteon (usan/inn)
26. Tasimelteon [vandf]
27. Tasimelteon [mart.]
28. Tasimelteon [who-dd]
29. Gtpl7393
30. Schembl3505912
31. Chembl2103822
32. Tasimelteon, >=98% (hplc)
33. Amy6925
34. Dtxsid70209826
35. Tasimelteon [orange Book]
36. Hms3885l17
37. Act06729
38. Bcp07180
39. Ex-a2729
40. Zinc4392649
41. Mfcd09033789
42. S4281
43. Akos025149360
44. Ac-6143
45. Ccg-266915
46. Cs-5512
47. Db09071
48. Ncgc00522560-01
49. As-35291
50. Hy-14803
51. T3813
52. J3.640.465e
53. D09388
54. 799t226
55. Q7687250
56. N-((2-(2,3-dihydro-4-benzofuranyl)cyclopropyl)methyl)propanamide
57. (trans)-n-[[2-(2,3-dihydrobenzofuran-4-yl) Cyclopropyl]methyl]propanamide
58. (trans)-n-[[2-(2,3-dihydrobenzofuran-4-yl)cyclopropyl]methyl]propanamide
59. (-)-(trans)-n-[[2-(2,3-dihydrobenzofuran-4-yl)cycloprop-1-yl] Methyl]propanamide
| Molecular Weight | 245.32 g/mol |
|---|---|
| Molecular Formula | C15H19NO2 |
| XLogP3 | 2.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 4 |
| Exact Mass | 245.141578849 g/mol |
| Monoisotopic Mass | 245.141578849 g/mol |
| Topological Polar Surface Area | 38.3 Ų |
| Heavy Atom Count | 18 |
| Formal Charge | 0 |
| Complexity | 318 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
| 1 of 2 | |
|---|---|
| Drug Name | Hetlioz |
| PubMed Health | Tasimelteon (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Drug Label | HETLIOZ (tasimelteon) is a melatonin receptor agonist, chemically designated as (1R, 2R)-N-[2-(2,3-dihydrobenzofuran-4-yl)cyclopropylmethyl]propanamide, containing two chiral centers. The molecular formula is C15H19NO2, and the molecular weight is 24... |
| Active Ingredient | Tasimelteon |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 20mg |
| Market Status | Prescription |
| Company | Vanda Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Hetlioz |
| PubMed Health | Tasimelteon (By mouth) |
| Drug Classes | Central Nervous System Agent |
| Drug Label | HETLIOZ (tasimelteon) is a melatonin receptor agonist, chemically designated as (1R, 2R)-N-[2-(2,3-dihydrobenzofuran-4-yl)cyclopropylmethyl]propanamide, containing two chiral centers. The molecular formula is C15H19NO2, and the molecular weight is 24... |
| Active Ingredient | Tasimelteon |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | 20mg |
| Market Status | Prescription |
| Company | Vanda Pharms |
Tasimelteon is indicated for the treatment of Non-24-Hour Sleep-Wake Disorder (N24HSWD).
FDA Label
Hetlioz is indicated for the treatment of Non-24-Hour Sleep-Wake Disorder (Non-24) in totally blind adults.
N05CH
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CH - Melatonin receptor agonists
N05CH03 - Tasimelteon
Route of Elimination
Following oral administration of radiolabeled tasimelteon, 80% of total radioactivity was excreted in urine and approximately 4% in feces, resulting in a mean recovery of 84%. Less than 1% of the dose was excreted in urine as the parent compound.
Volume of Distribution
The apparent oral volume of distribution of tasimelteon at steady state in young healthy subjects is approximately 56 - 126 L.
Tasimelteon is extensively metabolized. Metabolism of tasimelteon consists primarily of oxidation at multiple sites and oxidative dealkylation resulting in opening of the dihydrofuran ring followed by further oxidation to give a carboxylic acid. CYP1A2 and CYP3A4 are the major isozymes involved in the metabolism of tasimelteon. Phenolic glucuronidation is the major phase II metabolic route.
The observed mean elimination half-life for tasimelteon is 1.3 0.4 hours.
Tasimelteon is a selective dual agonist of the melatonin receptors MT1 and MT2.






