



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
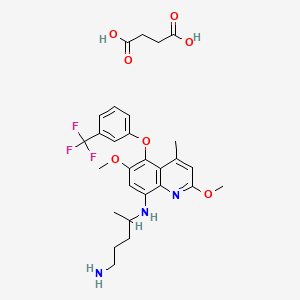

1. (r)-tafenoquine Succinate
2. Arakoda
3. Butanedioic Acid, Compd. With N4-(2,6-dimethoxy-4-methyl-5-(3-(trifluoromethyl)phenoxy)-8-quinolinyl)-1,4-pentanediamine (1:1)
4. Butanedioic Acid, Compd. With N4-(2,6-dimethoxy-4-methyl-5-(3-(trifluoromethyl)phenoxy)-8-quinolinyl)-1,4-pentanediamine (1:1), (r)-
5. Krintafel
6. N(4)-(2,6-dimethoxy-4-methyl-5-((3-trifluoromethyl)phenoxy)-8-quinolinyl)-1,4-pentanediamine
7. Sb-252263-ax
8. Sb-252263ax
9. Sb252263-ax
10. Sb252263ax
11. Tafenoquine
12. Tafenoquine Maleate
13. Tafenoquine Succinate, (r)-
14. Wr 238605
15. Wr-238605
16. Wr238605
1. 106635-81-8
2. Wr-238605
3. Tafenoquine Succinate [usan]
4. Tafenoquine (succinate)
5. Dl5j0b8vss
6. 106635-81-8 (succinate)
7. Arakoda
8. Krintafel
9. Sb-252263-ax
10. Wr238605 Succinate
11. Butanedioic Acid, Compd. With N4-[2,6-dimethoxy-4-methyl-5-[3-(trifluoromethyl)phenoxy]-8-quinolinyl]-1,4-pentanediamine (1:1)
12. Tafenoquine Succinate (usan)
13. N4-(2,6-dimethoxy-4-methyl-5-(3-(trifluoromethyl)phenoxy)quinolin-8-yl)pentane-1,4-diamine Succinate
14. N4-(2,6-dimethoxy-4-methyl-5-(3-(trifluoromethyl)phenoxy)quinolin-8-yl)pentane-1,4-diamine Succinate(1:1)
15. Unii-dl5j0b8vss
16. Sb-252263
17. Krintafel (tn)
18. Butanedioic Acid, Compd. With N4-(2,6-dimethoxy-4-methyl-5-(3-(trifluoromethyl)phenoxy)-8-quinolinyl)-1,4-pentanediamine (1:1)
19. Arakoda (tn)
20. Wr 238605 Succinate
21. Butanedioic Acid, Compd. With N(sup 4)-(2,6-dimethoxy-4-methyl-5-(3-(trifluoromethyl)phenoxy)-8-quinolinyl)-1,4-pentanediamine (1:1)
22. Mls006010680
23. Wr 238605 (succinate)
24. Schembl1004186
25. Chembl2364635
26. Dtxsid10910065
27. Tafenoquine Succinate [mi]
28. Akos037648507
29. Tafenoquine Succinate [who-dd]
30. Hy-111529a
31. Tafenoquine Succinate, >=95% (hplc)
32. Bs-14295
33. Butanedioic Acid;4-n-[2,6-dimethoxy-4-methyl-5-[3-(trifluoromethyl)phenoxy]quinolin-8-yl]pentane-1,4-diamine
34. Smr004701662
35. Tafenoquine Succinate [orange Book]
36. Cs-0083862
37. D10670
38. D80913
39. A904698
40. Q27276464
41. (4rs)-n4-(2,6-dimethoxy-4-methyl-5-(3-(trifluoromethyl)phenoxy)quinolin-8-yl)pentane-1,4-diamine Butanedioate
42. 8-[(4-amino-1-methylbutyl)amino]-2,6-dimethoxy-4-methyl-5-(3-trifluoromethylphenoxy)quinoline Succinate
43. 8-[(4-amino-1-methylbutyl)amino]-2,6-dimethoxy-4-methyl-5-[3-(trifluoromethyl)phenoxy]quinoline Succinate
44. N4-(2,6-dimethoxy-4-methyl-5-(3-(trifluoromethyl)phenoxy)quinolin-8-yl)pentane-1,4-diaminesuccinate(1:1)
| Molecular Weight | 581.6 g/mol |
|---|---|
| Molecular Formula | C28H34F3N3O7 |
| Hydrogen Bond Donor Count | 4 |
| Hydrogen Bond Acceptor Count | 13 |
| Rotatable Bond Count | 12 |
| Exact Mass | 581.23488492 g/mol |
| Monoisotopic Mass | 581.23488492 g/mol |
| Topological Polar Surface Area | 153 Ų |
| Heavy Atom Count | 41 |
| Formal Charge | 0 |
| Complexity | 690 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 1 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antimalarials
Agents used in the treatment of malaria. They are usually classified on the basis of their action against plasmodia at different stages in their life cycle in the human. (From AMA, Drug Evaluations Annual, 1992, p1585) (See all compounds classified as Antimalarials.)


