



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
Annual Reports
0
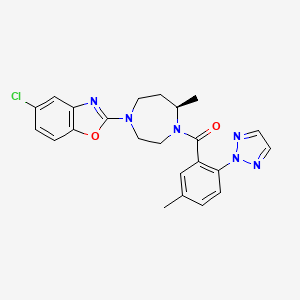

1. (4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl)(5-methyl-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone
2. Belsomra
3. Methanone, ((7r)-4-(5-chloro-2-benzoxazolyl)hexahydro-7-methyl-1h-1,4-diazepin-1-yl)(5-methyl-2-(2h-1,2,3-triazol-2-yl)phenyl)-
4. Mk 4305
5. Mk-4305
6. Mk4305
1. 1030377-33-3
2. Mk-4305
3. Belsomra
4. Mk4305
5. Mk 4305
6. [(7r)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl][5-methyl-2-(2h-1,2,3-triazol-2-yl)phenyl]methanone
7. Suvorexant (mk-4305)
8. 5-chloro-2-[(5r)-5-methyl-4-[5-methyl-2-(2h-1,2,3-triazol-2-yl)benzoyl]-1,4-diazepan-1-yl]-1,3-benzoxazole
9. Chembl1083659
10. Chebi:82698
11. 081l192fo9
12. ((7r)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl)(5-methyl-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone
13. Methanone, ((7r)-4-(5-chloro-2-benzoxazolyl)hexahydro-7-methyl-1h-1,4-diazepin-1-yl)(5-methyl-2-(2h-1,2,3-triazol-2-yl)phenyl)-
14. (r)-(4-(5-chlorobenzo[d]oxazol-2-yl)-7-methyl-1,4-diazepan-1-yl)(5-methyl-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone.
15. [(7r)-4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl]-[5-methyl-2-(triazol-2-yl)phenyl]methanone
16. Suvorexant [usan:inn]
17. Dora-analogue
18. Unii-081l192fo9
19. Belsomra (tn)
20. Suv
21. Suvorexant [mi]
22. Suvorexant [inn]
23. Suvorexant [jan]
24. Suvorexant (jan/usan)
25. Suvorexant [usan]
26. Suvorexant; Mk 4305
27. Suvorexant [vandf]
28. Suvorexant [who-dd]
29. Amy337
30. Gtpl2890
31. Schembl1586289
32. Suvorexant [orange Book]
33. Dtxsid90145616
34. Ex-a211
35. Belsomra Component Suvorexant
36. Bdbm50318701
37. Mfcd22377755
38. Zinc49036447
39. Akos022185167
40. Cs-0614
41. Db09034
42. Suvorexant Component Of Belsomra
43. (4-(5-chloro-1,3-benzoxazol-2-yl)-7-methyl-1,4-diazepan-1-yl)(5-methyl-2-(2h-1,2,3-triazol-2-yl)phenyl)methanone
44. Ac-30276
45. As-74879
46. Hy-10807
47. Bcp0726000197
48. Ft-0697203
49. Sw219649-1
50. D10082
51. J-690010
52. Q7650517
53. Cas:1030377-33-3;mk-4305
54. 5-chloro-2-[(5r)-5-methyl-4-[5-methyl-2-(2h-1,2,3-triazol-2-yl)benzoyl]-1,4-diazepan-1-yl]-1,3-benzoxazole Mk 4305
| Molecular Weight | 450.9 g/mol |
|---|---|
| Molecular Formula | C23H23ClN6O2 |
| XLogP3 | 4.9 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 3 |
| Exact Mass | 450.1571017 g/mol |
| Monoisotopic Mass | 450.1571017 g/mol |
| Topological Polar Surface Area | 80.3 Ų |
| Heavy Atom Count | 32 |
| Formal Charge | 0 |
| Complexity | 664 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Suvorexant is indicated for the treatment of insomnia characterized by difficulties with sleep onset and/or sleep maintenance.
FDA Label
Orexin Receptor Antagonists
Substances that bind to and inhibit the action of OREXIN RECEPTORS. Drugs in this class have been used as SLEEP AIDS, PHARMACEUTICAL. (See all compounds classified as Orexin Receptor Antagonists.)
Sleep Aids, Pharmaceutical
Drugs used to induce SLEEP, prevent SLEEPLESSNESS, or treat SLEEP INITIATION AND MAINTENANCE DISORDERS. (See all compounds classified as Sleep Aids, Pharmaceutical.)
N - Nervous system
N05 - Psycholeptics
N05C - Hypnotics and sedatives
N05CM - Other hypnotics and sedatives
N05CM19 - Suvorexant
Absorption
Peak concentrations occur at a median Tmax of 2 hours under fasted conditions. Ingestion of suvorexant with a high-fat meal has no effect on AUC or Cmax, but may delay Tmax by approximately 1.5 hours. Mean absolute bioavailability of 10 mg is 82%.
Route of Elimination
Approximately 66% is eliminated in feces and 23% is eliminated in urine.
Volume of Distribution
Mean volume of distribution is approximately 49 litres.
Suvorexant is primarily metabolized by cytochrome-P450 3A4 enzyme (CYP3A4) with a minor contribution from CYP2C19. Major circulating metabolites are suvorexant and a hydroxy-suvorexant metabolite, which is not expected to be pharmacologically active. There is potential for drug-drug interactions with drugs that inhibit or induce CYP3A4 activity.
Mean half life is approximately 12 hours.
Suvorexant is a dual antagonist of orexin receptors OX1R and OX2R. It exerts its pharmacological effect by inhibiting binding of neuropeptides orexin A and B, also known as hypocretin 1 and 2, that are produced by neurons in the lateral hypothalamus. These neurons control the wake-promoting centers of the brain and are active during wakefulness, especially during motor activities, and stop firing during sleep. By inhibiting the reinforcement of arousal systems, suvorexant use causes a decrease in arousal and wakefulness, rather than having a direct sleep-promoting effect.






