



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
Other Certificates
Other Suppliers

USA (Orange Book)

Europe

Canada

Australia

South Africa
Uploaded Dossiers
U.S. Medicaid
Annual Reports
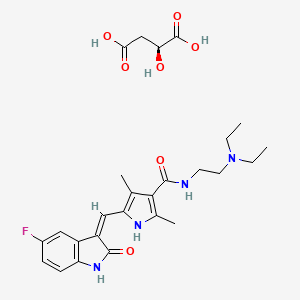

1. 5-(5-fluoro-2-oxo-1,2-dihydroindolylidenemethyl)-2,4-dimethyl-1h-pyrrole-3-carboxylic Acid (2-diethylaminoethyl)amide
2. Su 011248
3. Su 11248
4. Su-011248
5. Su-11248
6. Su011248
7. Su11248
8. Sunitinib
9. Sutent
1. 341031-54-7
2. Sutent
3. Su011248 L-malate Salt
4. Sunitinib Malate [usan]
5. Pha-290940ad
6. Su010398
7. Sunitinib L-malate
8. Su-011248 L-malate Salt
9. Lvx8n1ut73
10. Su-010398
11. (z)-n-(2-(diethylamino)ethyl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1h-pyrrole-3-carboxamide (s)-2-hydroxysuccinate
12. Su 11248
13. N-[2-(diethylamino)ethyl]-5-[(z)-(5-fluoro-2-oxo-1h-indol-3-ylidene)methyl]-2,4-dimethyl-1h-pyrrole-3-carboxamide;(2s)-2-hydroxybutanedioic Acid
14. Butanedioic Acid, Hydroxy-, (2s)-, Compd. With N-(2-(diethylamino)ethyl)-5-((z)-(5-fluoro-1,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl)-2,4-dimethyl-1h-pyrrole-3-carboxamide (1:1)
15. N-(2-(diethylamino)ethyl)-5-((z)-(5-fluoro-1,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl)-2,4-dimethyl-1h-pyrrole-3-carboxamide (2s)-hydroxybutanedioate
16. (2s)-2-hydroxybutanedioic Acid; N-[2-(diethylamino)ethyl]-5-{[(3z)-5-fluoro-2-oxo-2,3-dihydro-1h-indol-3-ylidene]methyl}-2,4-dimethyl-1h-pyrrole-3-carboxamide
17. Butanedioic Acid, 2-hydroxy-, (2s)-, Compd. With N-[2-(diethylamino)ethyl]-5-[(z)-(5-fluoro-1,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl]-2,4-dimethyl-1h-pyrrole-3-carboxamide (1:1)
18. Su 011248
19. Unii-lvx8n1ut73
20. Butanedioic Acid, 2-hydroxy-, (2s)-, Compd. With N-(2-(diethylamino)ethyl)-5-((z)-(5-fluoro-1,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl)-2,4-dimethyl-1h-pyrrole-3-carboxamide (1:1)
21. Mfcd08282795
22. Pdgf Tk Antagonist
23. Sutent (tn)
24. Sunitinib Malate- Bio-x
25. Sutent (tn) (pfizer)
26. Sunitinib Malate (sutent)
27. Chembl1567
28. Su 11248 (malate)
29. Su-11248 L-malate Salt
30. Sunitinib Malate [mi]
31. Sunitinib Malate (jan/usan)
32. Sunitinib Malate [jan]
33. Schembl1258610
34. Schembl1772213
35. Dtxsid4046492
36. Sunitinib Malate [vandf]
37. Sunitinib Malate [mart.]
38. Pnu-290940ad
39. Sunitinib Malate [who-dd]
40. Hms3261h09
41. Sunitinib Malate, >=98% (hplc)
42. Tox21_500514
43. Nsc736511
44. S1042
45. Su-11248j
46. Akos005145765
47. Akos025149097
48. Sunitinib Malate (sunitinib L-malate)
49. Sunitinib Malate [orange Book]
50. Ccg-221818
51. Cs-0177
52. Ks-5023
53. Ncgc00261199-01
54. Bs164428
55. Hy-10255
56. Am20090634
57. Sw219407-1
58. D06402
59. 031s547
60. A822079
61. J-019449
62. Q27283213
63. Sunitinib Malate
64. 341031-54-7
65. Sutent
66. Su-11248
67. Sunitinib
68. (2s)-2-hydroxybutanedioic Acid Compd. With N-[2-(diethylamino)ethyl]-5-[(z)-(5-fluoro-1,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl]-2,4-dimethyl-1h-pyrrole-3-carboxamide (1:1)
69. (z)-n-(2-(diethylamino)ethyl)-5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1h-pyrrole-3-carboxamide(s)-2-hydroxysuccinate
70. 1h-pyrrole-3-carboxamide, N-(2-(diethylamino)ethyl)-5-((z)-(5-fluoro-1,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl)-2,4-dimethyl-, (2s)-hydroxybutanedioate (1:1)
71. 1h-pyrrole-3-carboxamide,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl]-2,4-dimethyl-, (2s)-hydroxybutanedioate (1:1)
72. 1h-pyrrole-3-carboxamide,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl]-2,4-dimethyl-, Malate Salt
73. Butanedioic Acid, (2s)-, Compd. With N-[2-(diethylamino)ethyl]-5-[(z)-(5-fluoro-1,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl]-2,4-dimethyl-1h-pyrrole-3-carboxamide (1:1)
74. Butanedioic Acid, (2s)-, Compd. With N-[2-(diethylamino)ethyl]-5-[(z)-(5-fluoro-1,2-dihydro-2-oxo-3h-indol-3-ylidene)methyl]-2,4-dimethyl-1h-pyrrole-3-carboxamide (1:1) (ca Index Name)
75. N-(2-diethylamino)ethyl)-5-((z)-(5-fluoro-2-oxo-1,2-dihydro-3h-indol-3-ylidene)methyl)-2,4-dimethyl-1h-pyrrole-3-carboxamide Hydrogen (2s)-2-hydroxybutanedioate
76. N-[2-(diethylamino)ethyl]-5-[(z)-(5-fluoro-1,2-dihydro-2-oxo-3h-indol-3-ylidine)methyl]-2,4-dimethyl-1h-pyrrole-3-carboxamide (2s)-2-hydroxybutanedioate Salt
| Molecular Weight | 532.6 g/mol |
|---|---|
| Molecular Formula | C26H33FN4O7 |
| Hydrogen Bond Donor Count | 6 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 10 |
| Exact Mass | 532.23332757 g/mol |
| Monoisotopic Mass | 532.23332757 g/mol |
| Topological Polar Surface Area | 172 Ų |
| Heavy Atom Count | 38 |
| Formal Charge | 0 |
| Complexity | 765 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 1 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 2 | |
|---|---|
| Drug Name | Sunitinib malate |
| Drug Label | SUTENT, an oral multi-kinase inhibitor, is the malate salt of sunitinib. Sunitinib malate is described chemically as Butanedioic acid, hydroxy-, (2S)-, compound with N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)meth... |
| Active Ingredient | Sunitinib malate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 50mg base; eq 12.5mg base; eq 25mg base; eq 37.5mg base |
| Market Status | Prescription |
| Company | Mylan Pharms |
| 2 of 2 | |
|---|---|
| Drug Name | Sunitinib malate |
| Drug Label | SUTENT, an oral multi-kinase inhibitor, is the malate salt of sunitinib. Sunitinib malate is described chemically as Butanedioic acid, hydroxy-, (2S)-, compound with N-[2-(diethylamino)ethyl]-5-[(Z)-(5-fluoro-1,2-dihydro-2-oxo-3H-indol-3-ylidine)meth... |
| Active Ingredient | Sunitinib malate |
| Dosage Form | Capsule |
| Route | Oral |
| Strength | eq 50mg base; eq 12.5mg base; eq 25mg base; eq 37.5mg base |
| Market Status | Prescription |
| Company | Mylan Pharms |
* Gastrointestinal stromal tumour (GIST):
Sutent is indicated for the treatment of unresectable and/or metastatic malignant gastrointestinal stromal tumour (GIST) in adults after failure of imatinib mesilate treatment due to resistance or intolerance.
* Metastatic renal cell carcinoma (MRCC):
Sutent is indicated for the treatment of advanced/metastatic renal cell carcinoma (MRCC) in adults.
* Pancreatic neuroendocrine tumours (pNET):
Sutent is indicated for the treatment of unresectable or metastatic, well-differentiated pancreatic neuroendocrine tumours with disease progression in adults.
Experience with Sutent as first-line treatment is limited (see section 5. 1).
Angiogenesis Inhibitors
Agents and endogenous substances that antagonize or inhibit the development of new blood vessels. (See all compounds classified as Angiogenesis Inhibitors.)
Antineoplastic Agents
Substances that inhibit or prevent the proliferation of NEOPLASMS. (See all compounds classified as Antineoplastic Agents.)
Protein Kinase Inhibitors
Agents that inhibit PROTEIN KINASES. (See all compounds classified as Protein Kinase Inhibitors.)
L01EX01






