


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
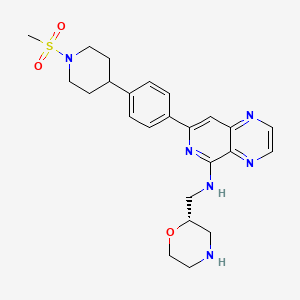

1. Sovleplenib [inn]
2. 9cl6353kho
3. 1415792-84-5
4. (s)-7-(4-(1-(methylsulfonyl)piperidin-4-yl)phenyl)-n-(morpholin-2-ylmethyl)pyrido(4,3-b)pyrazin-5-amine
5. Pyrido[3,4-b]pyrazin-5-amine, 7-[4-[1-(methylsulfonyl)-4-piperidinyl]phenyl]-n-[(2s)-2-morpholinylmethyl]-
6. Unii-9cl6353kho
7. Hmpl523
8. Hmpl-523
9. Hmpl-523 [who-dd]
10. Schembl14242080
11. Gtpl11886
12. Zinc143715528
13. Compound 225 [wo2012167733a1]
14. Hy-145598
15. Cs-0376654
16. F78019
17. (s)-7-(4-(1-(methylsulfonyl)piperidin-4-yl)phenyl)-n-(morpholin-2-ylmethyl)pyrido[3,4-b]pyrazin-5-amine
| Molecular Weight | 482.6 g/mol |
|---|---|
| Molecular Formula | C24H30N6O3S |
| XLogP3 | 1.2 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Exact Mass | 482.21001001 g/mol |
| Monoisotopic Mass | 482.21001001 g/mol |
| Topological Polar Surface Area | 118 Ų |
| Heavy Atom Count | 34 |
| Formal Charge | 0 |
| Complexity | 748 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 1 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


