



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0

1. (r*,r*)-(+-)-2,3-dihydroxybutanedioic Acid, Monoammonium Monosodium Salt
2. Aluminum Tartrate
3. Ammonium Tartrate
4. Calcium Tartrate
5. Calcium Tartrate Tetrahydrate
6. Mn(iii) Tartrate
7. Potassium Tartrate
8. Seignette Salt
9. Sodium Ammonium Tartrate
10. Sodium Potassium Tartrate
11. Sodium Tartrate
12. Stannous Tartrate
13. Tartaric Acid
14. Tartaric Acid, ((r*,r*)-(+-))-isomer
15. Tartaric Acid, (r*,s*)-isomer
16. Tartaric Acid, (r-(r*,r*))-isomer
17. Tartaric Acid, (s-(r*,r*))-isomer
18. Tartaric Acid, Ammonium Sodium Salt, (1:1:1) Salt, (r*,r*)-(+-)-isomer
19. Tartaric Acid, Calcium Salt, (r-r*,r*)-isomer
20. Tartaric Acid, Monoammonium Salt, (r-(r*,r*))-isomer
21. Tartrate
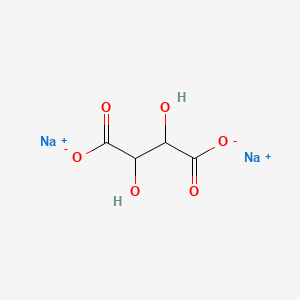
1. Sodium Tartrate
2. 868-18-8
3. Disodium;2,3-dihydroxybutanedioate
4. Sodium 2,3-dihydroxysuccinate
5. 51307-92-7
6. Disodium 2,3-dihydroxybutanedioate
7. Disodium Tartrate Solution
8. Sodium-tartrate
9. Sodium Tartarate
10. Butanedioic Acid, 2,3-dihydroxy-, Sodium Salt (1:2), (2r,3r)-rel-
11. Sodium Tartrate Dibasic Solution
12. Sodium (2r,3r)-2,3-dihydroxysuccinate(x:1)
13. Butanedioic Acid, 2,3-dihydroxy-, Monosodium Salt, (2r,3r)-rel-
14. 14475-11-7
15. Schembl24913
16. Dl-tartaric Acid Disodium Salt
17. Chembl3917380
18. Sodium (dl)-(+/-)-tartrate
19. Akos006220952
20. Sb47841
21. As-56692
22. Db-056954
23. Ft-0632296
24. Ft-0633039
25. Sodium Tartrate Dibasic Solution, P.a., 99.5%
26. Q283499
27. Sodium Tartrate Dibasic Solution, Bioultra, 1.5 M In H2o (colorless Solution At 20 C)
| Molecular Weight | 194.05 g/mol |
|---|---|
| Molecular Formula | C4H4Na2O6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 6 |
| Rotatable Bond Count | 1 |
| Exact Mass | 193.98032641 g/mol |
| Monoisotopic Mass | 193.98032641 g/mol |
| Topological Polar Surface Area | 121 Ų |
| Heavy Atom Count | 12 |
| Formal Charge | 0 |
| Complexity | 123 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 2 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 3 |
A - Alimentary tract and metabolism
A06 - Drugs for constipation
A06A - Drugs for constipation
A06AD - Osmotically acting laxatives
A06AD21 - Sodium tartrate


