
Synopsis
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
NDC API
0
VMF
0
Listed Suppliers
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Europe
0
Australia
0
South Africa
0
Listed Dossiers
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
API
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
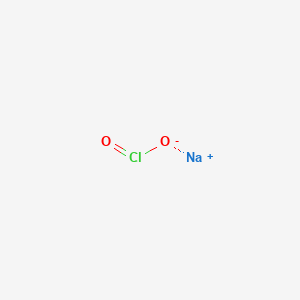

1. Chlorite
1. 7758-19-2
2. Chlorous Acid, Sodium Salt
3. Textone
4. Sodium;chlorite
5. Chlorite Sodium
6. Naclo2
7. G538ebv4vf
8. Chebi:78667
9. Sodium Chlorite (water Disinfection Byproducts)
10. Textile
11. Alcide Ld
12. Neo Silox D
13. Caswell No. 755
14. Sodiumchlorite
15. Ccris 1426
16. Hsdb 733
17. Chlorite (sodium Salt)
18. Einecs 231-836-6
19. Un1496
20. Unii-g538ebv4vf
21. Epa Pesticide Chemical Code 020502
22. Chlorous Acid, Sodium Salt (1:1)
23. Nao2cl
24. Dsstox_cid_1272
25. Ec 231-836-6
26. Dsstox_rid_76050
27. Dsstox_gsid_21272
28. Sodium Chlorite [mi]
29. Sodium Chlorite [hsdb]
30. Sodium Chlorite [iarc]
31. Sodium Chlorite [inci]
32. Chembl1887585
33. Dtxsid8021272
34. Sodium Chlorite [who-dd]
35. Tox21_302800
36. Mfcd00003478
37. Akos015843819
38. Db13210
39. Ncgc00091419-01
40. Ncgc00256359-01
41. Sodium Chlorite [un1496] [oxidizer]
42. Cas-7758-19-2
43. Ft-0695293
44. C19523
45. Q411294
| Molecular Weight | 90.44 g/mol |
|---|---|
| Molecular Formula | ClNaO2 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 89.9484512 g/mol |
| Monoisotopic Mass | 89.9484512 g/mol |
| Topological Polar Surface Area | 40.1 Ų |
| Heavy Atom Count | 4 |
| Formal Charge | 0 |
| Complexity | 13.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
/VET/ Sodium chlorite is used in veterinary medicine as a medical disinfectant in post-milking teat dip products used topically for the control of mastitis in dairy cattle.
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines Evaluation Unit, Committee for Veterinary Medicinal Products; Sodium Chlorite, Summary Report. Available from, as of July 29, 2008: https://www.emea.europa.eu/pdfs/vet/mrls/sodiumchlorite.pdf
D - Dermatologicals
D03 - Preparations for treatment of wounds and ulcers
D03A - Cicatrizants
D03AX - Other cicatrizants
D03AX11 - Sodium chlorite
... The dermal absorption /was reported on/ Alcide, an antimicrobial compound consisting of solutions of sodium chlorite and lactic acid, which when mixed immediately before use result in the formation of chlorine dioxide. 0.6 g (36)Cl-labeled sodium chlorite as part of the Alcide was used to monitor absorption following application to the shaved backs of 10 female Sprague-Dawley rats. Maximum absorption of (36)Cl into plasma was observed after 72 hours, where a plasma concentration of 69.4 ug% (36)Cl was reached. The absorption half-life was calculated to be 22.1 hours, which corresponds to a rate constant of 0.0314 hr-1. /Alcide/
USEPA; TOXICOLOGICAL REVIEW OF CHLORINE DIOXIDE AND CHLORITE (CAS Nos. 10049-04-4 and 7758-19-2) In Support of Summary Information on the Integrated Risk Information System (IRIS) p.4 (September 2000) EPA/635/R-00/007. Available from, as of July 31, 2008: https://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showSubstanceList
... Sodium chlorite /was/ shown to be well absorbed by the oral route in rats, by using radiolabelled compound (Cmax 1-2 hours). ... The main route of excretion ... appeared to be via the kidneys, predominantly as chloride (32% of the dose at 72 hours), with some chloride (32% of the dose at 72 hours) and a little chlorate (0.73% of the dose at 72 hours). ... 83% of the recovered dose was found in urine, and 13% in feces. ...
European Medicines Agency (EMEA), The European Agency for the Evaluation of Medicinal Products, Veterinary Medicines Evaluation Unit, Committee for Veterinary Medicinal Products; Sodium Chlorite, Summary Report. Available from, as of July 29, 2008: https://www.emea.europa.eu/pdfs/vet/mrls/sodiumchlorite.pdf
Registrant Name : HPNC Co., Ltd.
Registration Date : 2019-03-26
Registration Number : Su472-1-ND
Manufacturer Name : Brenntag UK Ltd@Ercros SA
Manufacturer Address : Alpha House, Lawnswood Business Park, Redver Close Leeds, LS16 6QY, United Kingdom@Er...

 FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
FULL SCREEN VIEW Click here to open all results in a new tab [this preview display 10 results]
API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
34
PharmaCompass offers a list of Chlorite API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Chlorite manufacturer or Chlorite supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Chlorite manufacturer or Chlorite supplier.
PharmaCompass also assists you with knowing the Chlorite API Price utilized in the formulation of products. Chlorite API Price is not always fixed or binding as the Chlorite Price is obtained through a variety of data sources. The Chlorite Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A SODIUM CHLORITE manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of SODIUM CHLORITE, including repackagers and relabelers. The FDA regulates SODIUM CHLORITE manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. SODIUM CHLORITE API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
A SODIUM CHLORITE supplier is an individual or a company that provides SODIUM CHLORITE active pharmaceutical ingredient (API) or SODIUM CHLORITE finished formulations upon request. The SODIUM CHLORITE suppliers may include SODIUM CHLORITE API manufacturers, exporters, distributors and traders.
click here to find a list of SODIUM CHLORITE suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A SODIUM CHLORITE DMF (Drug Master File) is a document detailing the whole manufacturing process of SODIUM CHLORITE active pharmaceutical ingredient (API) in detail. Different forms of SODIUM CHLORITE DMFs exist exist since differing nations have different regulations, such as SODIUM CHLORITE USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A SODIUM CHLORITE DMF submitted to regulatory agencies in the US is known as a USDMF. SODIUM CHLORITE USDMF includes data on SODIUM CHLORITE's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The SODIUM CHLORITE USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of SODIUM CHLORITE suppliers with USDMF on PharmaCompass.
In Korea, the Ministry of Food and Drug Safety (MFDS) is in charge of regulating pharmaceutical products and services.
Pharmaceutical companies submit a SODIUM CHLORITE Drug Master File in Korea (SODIUM CHLORITE KDMF) to the MFDS, which includes comprehensive information about the production, processing, facilities, materials, packaging, and testing of SODIUM CHLORITE. The MFDS reviews the SODIUM CHLORITE KDMF as part of the drug registration process and uses the information provided in the SODIUM CHLORITE KDMF to evaluate the safety and efficacy of the drug.
After submitting a SODIUM CHLORITE KDMF to the MFDS, the registered manufacturer can provide importers or distributors with the registration number without revealing confidential information to Korean business partners. Applicants seeking to register their SODIUM CHLORITE API can apply through the Korea Drug Master File (KDMF).
click here to find a list of SODIUM CHLORITE suppliers with KDMF on PharmaCompass.
SODIUM CHLORITE Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of SODIUM CHLORITE GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right SODIUM CHLORITE GMP manufacturer or SODIUM CHLORITE GMP API supplier for your needs.
A SODIUM CHLORITE CoA (Certificate of Analysis) is a formal document that attests to SODIUM CHLORITE's compliance with SODIUM CHLORITE specifications and serves as a tool for batch-level quality control.
SODIUM CHLORITE CoA mostly includes findings from lab analyses of a specific batch. For each SODIUM CHLORITE CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
SODIUM CHLORITE may be tested according to a variety of international standards, such as European Pharmacopoeia (SODIUM CHLORITE EP), SODIUM CHLORITE JP (Japanese Pharmacopeia) and the US Pharmacopoeia (SODIUM CHLORITE USP).

