



API Suppliers

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0

USA (Orange Book)

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
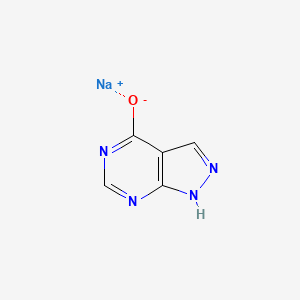

1. 17795-21-0
2. Sodium 1h-pyrazolo[3,4-d]pyrimidin-4-olate
3. Sodium Allopurinol
4. Allopurinol Sodium Salt
5. Aloprim
6. Sodium;1h-pyrazolo[3,4-d]pyrimidin-4-olate
7. Allopurinol Sodium (aloprim)
8. 1,5-dihydro-4h-pyrazolo(3,4-d)pyrimidin-4-one, Monosodium Salt
9. 428673rc2z
10. Einecs 241-771-5
11. Nsc 108836
12. Unii-428673rc2z
13. 1h-pyrazolo(3,4-d)pyrimidin-4-ol, Monosodium Salt
14. 4h-pyrazolo(3,4-d)pyrimidin-4-one, 1,5-dihydro-, Monosodium Salt
15. Allopurinolsodium
16. Schembl17223898
17. 1,5-dihydro-4h-pyrazolo[3,4-d]pyrimidin-4-one,monosodiumsalt
18. Akos015899245
19. Ac-4257
20. Sodium 1h-pyrazolo[3,4-d]pyrimidine-4-olate
21. F76872
22. A881435
23. Q27258510
| Molecular Weight | 158.09 g/mol |
|---|---|
| Molecular Formula | C5H3N4NaO |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 0 |
| Exact Mass | 158.02045501 g/mol |
| Monoisotopic Mass | 158.02045501 g/mol |
| Topological Polar Surface Area | 77.5 Ų |
| Heavy Atom Count | 11 |
| Formal Charge | 0 |
| Complexity | 136 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
| 1 of 4 | |
|---|---|
| Drug Name | Allopurinol sodium |
| PubMed Health | Allopurinol (Injection) |
| Drug Classes | Antigout, Urinary Stone Agent |
| Active Ingredient | Allopurinol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Eurohlth Intl |
| 2 of 4 | |
|---|---|
| Drug Name | Aloprim |
| Active Ingredient | Allopurinol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Mylan Institutional |
| 3 of 4 | |
|---|---|
| Drug Name | Allopurinol sodium |
| PubMed Health | Allopurinol (Injection) |
| Drug Classes | Antigout, Urinary Stone Agent |
| Active Ingredient | Allopurinol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Eurohlth Intl |
| 4 of 4 | |
|---|---|
| Drug Name | Aloprim |
| Active Ingredient | Allopurinol sodium |
| Dosage Form | Injectable |
| Route | Injection |
| Strength | eq 500mg base/vial |
| Market Status | Prescription |
| Company | Mylan Institutional |


