


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
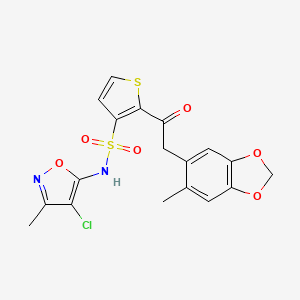

1. N-(4-chloro-3-methyl-5-isoxazolyl)-2-((4,5-(methylenedioxy)-o-toly)acetyl)-3-thiophenesulfonamide
2. N-(4-chloro-3-methyl-5-isoxazolyl)-2-(3,4-(methylenedioxy)-6-methyl)phenylacetyl-3-thiophenesulfonamide
3. Sitaxsentan
4. Tbc 11251
5. Tbc-11251
6. Tbc11251
1. Sitaxsentan
2. 184036-34-8
3. Tbc-11251
4. N-(4-chloro-3-methyl-1,2-oxazol-5-yl)-2-[2-(6-methyl-1,3-benzodioxol-5-yl)acetyl]thiophene-3-sulfonamide
5. Tbc11251
6. Ipi-1040
7. J9qh779mem
8. Chembl282724
9. N-(4-chloro-3-methyl-5-isoxazolyl)-2-((4,5-(methylenedioxy)-o-toly)acetyl)-3-thiophenesulfonamide
10. N-(4-chloro-3-methyl-5-isoxazolyl)-2-(3,4-(methylenedioxy)-6-methyl)phenylacetyl-3-thiophenesulfonamide
11. Tbc 11251
12. Sitaxentan [inn]
13. Ipi 1040
14. Sitaxentan [usan:inn]
15. Unii-j9qh779mem
16. Sitaxsentan [mi]
17. Sitaxentan (usan/inn)
18. Sitaxentan [usan]
19. Dsstox_cid_31462
20. Dsstox_rid_97348
21. Sitaxentan [who-dd]
22. Dsstox_gsid_57673
23. 3-thiophenesulfonamide, N-(4-chloro-3-methyl-5-isoxazolyl)-2-((6-methyl-1,3-benzodioxol-5-yl)acetyl)-
24. N-(4-chloro-3-methyl-5-isoxazolyl)-2-((2-methyl-4,5-methylenedioxyphenyl)acetyl)thiophene-3-sulfonamide
25. N-(4-chloro-3-methyl-5-isoxazolyl)-2-((3,4-(methylenedioxy)-6-methylphenyl)acetyl)-3-thiophenesulfonamide
26. Schembl803344
27. Gtpl3950
28. Dtxsid0057673
29. Chebi:135736
30. Bcp13848
31. Zinc1481831
32. Tox21_113725
33. Bdbm50058126
34. Zinc328577260
35. Cs-0402
36. Db06268
37. Sb19551
38. Ncgc00253587-01
39. Hy-76520
40. N-(4-chloro-3-methylisoxazol-5-yl)-2-(2-(6-methylbenzo-[d][1,3]dioxol-5-yl)acetyl)thiophene-3-sulfonamide
41. Db-065456
42. Tbc11251 / Tbc-11251
43. Cas-184036-34-8
44. Ft-0701871
45. D07171
46. A927076
47. Q905664
48. Sr-01000945028
49. Sr-01000945028-1
50. 2-[2-(6-methyl-benzo[1,3]dioxol-5-yl)-acetyl]-thiophene-3-sulfonic Acid (4-chloro-3-methyl-isoxazol-5-yl) Amide
51. 2-[2-(6-methyl-benzo[1,3]dioxol-5-yl)-acetyl]-thiophene-3-sulfonic Acid (4-chloro-3-methyl-isoxazol-5-yl)-amide
52. 2-[2-(6-methyl-benzo[1,3]dioxol-5-yl)-acetyl]-thiophene-3-sulfonic Acid (4-chloro-3-methyl-isoxazol-5-yl)-amide (sitaxsentan)
53. N- (4- Chloro- 3- Methyl- Oxazol- 5- Yl)- 2- [2- (6- Methylbenzo[1,3]dioxol- 5- Yl)acetyl]- Thiophene- 3- Sulfonamide
54. N-(4-chloro-3-methyl-1,2-oxazol-5-yl)-2-[2-(6-methyl-1,3-benzodioxol-5-yl)acetyl]thiophene-3-sulfonamide.
55. N-(4-chloro-3-methyl-1,2-oxazol-5-yl)-2-[2-(6-methyl-2h-1,3-benzodioxol-5-yl)acetyl]thiophene-3-sulfonamide
56. N-(4-chloro-3-methyl-5-isoxazolyl)-2-[(6-methyl-1,3-benzodioxol-5-yl) Acetyl]-3-thiophenesulphonamide
57. N-(4-chloro-3-methyloxazol-5-yl)-2-[2-(6-methylbenzo[1,3]dioxol-5-yl)acetyl]thiophene-3-sulfonamide
| Molecular Weight | 454.9 g/mol |
|---|---|
| Molecular Formula | C18H15ClN2O6S2 |
| XLogP3 | 3.7 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 9 |
| Rotatable Bond Count | 6 |
| Exact Mass | 454.0060062 g/mol |
| Monoisotopic Mass | 454.0060062 g/mol |
| Topological Polar Surface Area | 144 Ų |
| Heavy Atom Count | 29 |
| Formal Charge | 0 |
| Complexity | 720 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
Investigated for use/treatment in pulmonary hypertension, connective tissue diseases, hypertension, and congestive heart failure.
Sitaxentan belongs to a class of drugs known as endothelin receptor antagonists (ERAs). Patients with PAH have elevated levels of endothelin, a potent blood vessel constrictor, in their plasma and lung tissue. Sitaxentan blocks the binding of endothelin to its receptors, thereby negating endothelin's deleterious effects.
Endothelin Receptor Antagonists
Compounds and drugs that bind to and inhibit or block the activation of ENDOTHELIN RECECPTORS. (See all compounds classified as Endothelin Receptor Antagonists.)
C - Cardiovascular system
C02 - Antihypertensives
C02K - Other antihypertensives
C02KX - Antihypertensives for pulmonary arterial hypertension
C02KX03 - Sitaxentan
Absorption
70-100%
Route of Elimination
Renal (50 to 60%) Fecal (40 to 50%)
Hepatic (CYP2C9- and CYP3A4-mediated)
10 hours
Sitaxentan is a competitive antagonist of endothelin-1 at the endothelin-A (ET-A) and endothelin-B (ET-B) receptors. Under normal conditions, endothelin-1 binding of ET-A or ET-B receptors causes pulmonary vasoconstriction. By blocking this interaction, Sitaxentan decreases pulmonary vascular resistance. Sitaxentan has a higher affinity for ET-A than ET-B.


