



API Suppliers

US DMFs Filed

CEP/COS Certifications

JDMFs Filed
Other Certificates
Other Suppliers
0

USA (Orange Book)

Europe

Canada

Australia
0

South Africa
Uploaded Dossiers
U.S. Medicaid
0
Annual Reports
0
0
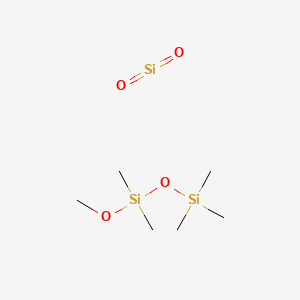

1. Phazyme 125
1. 8050-81-5
2. Dioxosilane;methoxy-dimethyl-trimethylsilyloxysilane
3. Simeticone
4. Schembl339371
5. Db09512
6. Ft-0674588
7. Q419415
1. Simethicone Usp
| Molecular Weight | 238.46 g/mol |
|---|---|
| Molecular Formula | C6H18O4Si3 |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 238.05128865 g/mol |
| Monoisotopic Mass | 238.05128865 g/mol |
| Topological Polar Surface Area | 52.6 Ų |
| Heavy Atom Count | 13 |
| Formal Charge | 0 |
| Complexity | 125 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Antifoaming Agents; Emollients
National Library of Medicine's Medical Subject Headings. Simethicone. Online file (MeSH, 2015). Available from, as of October 16, 2015: https://www.nlm.nih.gov/cgi/mesh/2015/MB_cgi
/EXPERIMENTAL THERAPY/ Currently, there is no standardized protocol for bowel preparation before small bowel capsule endoscopy (SBCE). This study aimed to investigate the effect of simethicone combined with polyethylene glycol (PEG) on the visualization quality (VQ) of the SBCE in patients with or without known or suspected Crohn's disease (CD). This observational, prospective, single-center study included consecutive patients undergoing a SBCE between 2007 and 2008. Patients received either a standard bowel cleansing preparation of 2 L PEG and 80 mg simethicone orally 12 and 1 h before SBCE respectively (Group A) or only PEG (Group B). VQ, based on scores for luminal bubbles in frames taken from the small intestine, examination completeness, SBCE diagnostic yield, gastric and small bowel transit times were recorded. Of the 115 patients finally included (Group A, n=56 and Group B, n=59) the cecum was visualized in 103 (89.6%). Simethicone overall improved the VQ in the proximal [OR: 2.43 (95%CI: 1.08-5.45), P=0.032] but not in the distal bowel segment (P=0.064). Nevertheless, this effect was not observed in patients undergoing SBCE for either known or suspected CD. Simethicone as an adjunct to PEG for bowel preparation in patients undergoing SBCE significantly improved the VQ in non-CD patients.
PMID:26423317 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4585393 Papamichael K et al; Ann Gastroenterol 28 (4): 464-8 (2015)
Simethicone is used as an adjunct in the symptomatic treatment of flatulence, functional gastric bloating, and postoperative gas pains. For self-medication, the drug is used as an antiflatulent to relieve symptoms commonly referred to as gas, including upper GI bloating, pressure, fullness, or stuffed feeling. Simethicone also has been used prior to gastroscopy to enhance visualization and prior to radiography of the intestine to reduce gas shadows. Although there is gastroscopic evidence that simethicone aids in the elimination of gas from the GI tract and reduces postoperative gas pains, the relationship of gas accumulation to what patients commonly refer to as symptoms of gas under ordinary conditions is not clear; however, the drug also has been shown to be effective in relieving these symptoms. Preparations of simethicone with antacids, antispasmodics, or digestive enzymes are available, but use of inflexible combinations of drugs is often unwarranted, and these products have not been well evaluated.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
Although simethicone is an effective antiflatulent, there currently is no conclusive evidence that immediate postprandial upper abdominal distress (IPPUAD) is caused by excessive gas, despite the fact that many patients commonly attribute symptoms of the distress to gas. In addition, current data are insufficient to establish the efficacy of simethicone for the symptomatic relief of IPPUAD, a symptom complex that occurs within 30 minutes after a meal and consists of sensations of GI bloating, distention, fullness, or pressure with upper abdominal discomfort but not aerophagia or hyperacidity.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
For more Therapeutic Uses (Complete) data for Simethicone (11 total), please visit the HSDB record page.
... Simethicone and carbamazepine, when taken together, may be a cause of carbamazepine toxicity. The risk of carbamazepine overdose should be considered when prescribing simethicone to a patient who is using carbamazepine.
PMID:18652684 Full text: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2495000 Guneysel O et al; J Med Case Reports 2: 242 (2008)
Although no data are available on the use of simethicone during breastfeeding, it is known that simethicone is not absorbed orally. Therefore, it cannot be transferred to breastmilk. It is also used safely in breastfed infants. No special precautions are required.
LacMed; Antifoaming Agents Record on Simethicone (8050-81-5) (LactMed Record 536) revised on September 7, 2013. Available from, as of October 22, 2015: https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/r?dbs+lactmed:@term+@DOCNO+536
Simethicone is apparently nontoxic, and no adverse effects have been reported.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
Simethicone is indicated for the treatment of bloating, pressure, and cramps caused by gas. Simethicone is also used as part of bowel preparation for colonoscopies.
Simethicone decreases the surface tension of gas bubbles in the gastrointestinal tract, facilitating their expulsion. It has a short duration of action as it is generally given as needed, and a wide therapeutic index as it is not systemically absorbed.
Emollients
Oleagenous substances used topically to soothe, soften or protect skin or mucous membranes. They are used also as vehicles for other dermatologic agents. (See all compounds classified as Emollients.)
Antifoaming Agents
Agents used to prevent the formation of foam or to treat flatulence or bloat. (See all compounds classified as Antifoaming Agents.)
Absorption
Simethicone is not systemically absorbed and so these data are not readily available.
Route of Elimination
Simethicone is eliminated in the feces.
Volume of Distribution
Simethicone is not systemically absorbed and so these data are not readily available.
Clearance
Simethicone is not systemically absorbed and so these data are not readily available.
Simethicone is physiologically inert; it does not appear to be absorbed from the GI tract or to interfere with gastric secretion or absorption of nutrients. Following oral administration, the drug is excreted unchanged in feces.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015
Simethicone is not systemically absorbed and so it is not metabolised by the body.
Simethicone is not systemically absorbed and so these data are not readily available.
Simethicone is a surfactant that decreases the surface tension of gas bubbles in the gastrointestinal tract, more easily allowing gas to exit the body.
The clinical use of simethicone is based on its antifoam properties. Silicone antifoams spread on the surface of aqueous liquids, forming a film of low surface tension and thus causing collapse of foam bubbles. Simethicone reportedly allows mucus-surrounded gas bubbles in the GI tract to coalesce and be expelled.
American Society of Health-System Pharmacists 2015; Drug Information 2015. Bethesda, MD. 2015


