



API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
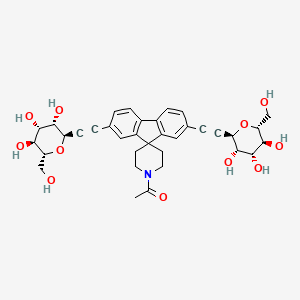

1. 1616113-45-1
2. Antibiotic-202
3. Vrt-1353385
4. Sibofimloc [inn]
5. Sibofimloc [usan]
6. Eb8018
7. 00of00qzc4
8. 1-[2,7-bis[2-[(2r,3s,4r,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]ethynyl]spiro[fluorene-9,4'-piperidine]-1'-yl]ethanone
9. 1-[2,7-bis[2-[(2r,3s,4r,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)tetrahydropyran-2-yl]ethynyl]spiro[fluorene-9,4'-piperidine]-1'-yl]ethanone
10. Eb-8018
11. 1-(2,7-bis(2,6-anhydro-7,8-dideoxy-d-glycero-d-manno-oct-7-ynitol-8-yl)spiro(fluorene-9,4'-piperidin)-1'-yl)ethan-1-one
12. 1-[2,7-bis({2-[(2r,3s,4r,5s,6r)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]ethynyl})spiro[fluorene-9,4'-piperidine]-1'-yl]ethan-1-one
13. 8,8'-(1'-acetylspiro(9h-fluorene-9,4'-piperidine)-2,7-diyl)bis(2,6-anhydro-7,8-dideoxy-d-glycero-d-manno-oct-7-ynitol)
14. D-glycero-d-manno-oct-7-ynitol, 8,8'-(1'-acetylspiro(9h-fluorene-9,4'-piperidine)-2,7-diyl)bis(2,6-anhydro-7,8-dideoxy-
15. Antibiotic 202
16. Sibofimloc (usan/inn)
17. Unii-00of00qzc4
18. Chembl4594305
19. Schembl15836795
20. Ex-a750
21. Antibiotic-202;vrt-1353385
22. Mfcd28144726
23. Who 10950
24. Zinc209678340
25. Cs-5487
26. Ac-35872
27. Da-43714
28. Hy-12820
29. B5896
30. D11825
31. J-690221
| Molecular Weight | 649.7 g/mol |
|---|---|
| Molecular Formula | C35H39NO11 |
| XLogP3 | -0.8 |
| Hydrogen Bond Donor Count | 8 |
| Hydrogen Bond Acceptor Count | 11 |
| Rotatable Bond Count | 4 |
| Exact Mass | 649.25231106 g/mol |
| Monoisotopic Mass | 649.25231106 g/mol |
| Topological Polar Surface Area | 201 Ų |
| Heavy Atom Count | 47 |
| Formal Charge | 0 |
| Complexity | 1190 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 10 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |


