



API Suppliers

US DMFs Filed

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
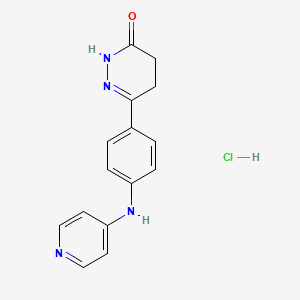

1. 6-(4-(4'-pyridyl)aminophenyl)-4,5-dihydro-3(2h)-pyridazinone Hydrochloride
2. Mci 154
3. Mci-154
1. 98326-33-1
2. Mci-154
3. Mci 154
4. Senazodan Hcl
5. Senazodan (hydrochloride)
6. 8aqj5mim3p
7. 6-(4-(pyridin-4-ylamino)phenyl)-4,5-dihydropyridazin-3(2h)-one Hydrochloride
8. Unii-8aqj5mim3p
9. 6-(4-(4'-pyridyl)aminophenyl)-4,5-dihydro-3(2h)-pyridazinone Hydrochloride
10. Chembl543966
11. Dtxsid00913328
12. Hy-101693a
13. 3(2h)-pyridazinone, 4,5-dihydro-6-(4-(4-pyridinylamino)phenyl)-, Monohydrochloride
14. 4,5-dihydro-6-(4-(4-pyridinylamino)phenyl)-3(2h)-pyridazinone Monohydrochloride
15. Cs-0033842
16. Q27270120
17. 3-[4-(pyridin-4-ylamino)phenyl]-4,5-dihydro-1h-pyridazin-6-one;hydrochloride
18. 3(2h)-pyridazinone, 4,5-dihydro-6-(4-(4-pyridinylamino)phenyl)-, Hydrochloride (1:1)
19. 6-{4-[(pyridin-4(1h)-ylidene)amino]phenyl}-4,5-dihydropyridazin-3-ol--hydrogen Chloride (1/1)
| Molecular Weight | 302.76 g/mol |
|---|---|
| Molecular Formula | C15H15ClN4O |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 3 |
| Exact Mass | 302.0934388 g/mol |
| Monoisotopic Mass | 302.0934388 g/mol |
| Topological Polar Surface Area | 66.4 Ų |
| Heavy Atom Count | 21 |
| Formal Charge | 0 |
| Complexity | 368 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 2 |
Cardiotonic Agents
Agents that have a strengthening effect on the heart or that can increase cardiac output. They may be CARDIAC GLYCOSIDES; SYMPATHOMIMETICS; or other drugs. They are used after MYOCARDIAL INFARCT; CARDIAC SURGICAL PROCEDURES; in SHOCK; or in congestive heart failure (HEART FAILURE). (See all compounds classified as Cardiotonic Agents.)
Vasodilator Agents
Drugs used to cause dilation of the blood vessels. (See all compounds classified as Vasodilator Agents.)


