
Synopsis
0
CEP/COS
0
JDMF
0
EU WC
0
KDMF
0
NDC API
0
VMF
0
EDQM
0
USP
0
JP
0
Others
0
FDA Orange Book
0
Australia
DRUG PRODUCT COMPOSITIONS
0
US Patents
0
US Exclusivities
0
Health Canada Patents
US Medicaid
NA
Annual Reports
NA
Regulatory FDF Prices
NA
0
FDF
0
Data Compilation #PharmaFlow
0
Stock Recap #PipelineProspector
0
Weekly News Recap #Phispers
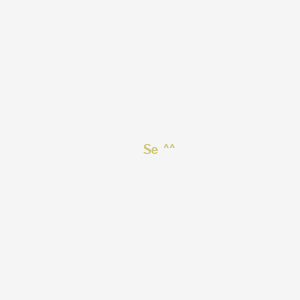
1. Hydrogen Selenide
2. Hydrogen Selenide, 75se-labeled
3. Selane
1. 7782-49-2
2. Se
3. Selane
4. Selenium Hydride
5. Selenium Atom
6. Elemental Selenium
7. Selenium Elemental
8. Selenium, Elemental
9. Selen
10. Selenium And Compounds
11. Selenium Dihydride
12. Selenium Metallicum
13. Selenium Powder
14. Selenium Compounds
15. Ci 77805
16. Dihydrogen Monoselenide
17. C.i. 77805
18. Mfcd00134090
19. Hydrogen Selenide, Anhydrous
20. H6241uj22b
21. Vandex
22. Gray Selenium
23. Selenium Alloy
24. Selenium Base
25. Selenium Dust
26. Colloidal Selenium
27. Selen [polish]
28. Selenium, Metallic
29. Selenium Homopolymer
30. Selenium, Colloidal
31. Caswell No. 732
32. Selenio
33. Selenium Anhydride
34. Electronic E-2
35. Ccris 4250
36. Hsdb 4493
37. Einecs 231-957-4
38. Un2658
39. Hydrogen Selenide (h2se)
40. Epa Pesticide Chemical Code 072001
41. Selenium Compounds, N.o.s.
42. Selenium Metal
43. Unii-h6241uj22b
44. Selenium And Selenium Compounds
45. Hsdb 548
46. Selenium (powder)
47. Einecs 231-978-9
48. Selenium Shot, 4n
49. Un2202
50. Un3283
51. Selenium (iv) Hydride
52. Hydrogen Selenid
53. B-traxim Se
54. Selenium [mi]
55. Selenium [vandf]
56. Selenium Hydride (seh2)
57. Selenium [mart.]
58. 34se
59. Selenium [who-dd]
60. Unii-v91p54kpam
61. Ec 231-957-4
62. Selenium, Powder, 99+%
63. Selenium Compound, N.o.s.
64. Selenium Powder, -200 Mesh
65. Selenium Powder, -325 Mesh
66. Selsaf 2000
67. Hydrogen Selenide [mi]
68. Selenium, P.a., 99.5%
69. Chembl1235891
70. Dtxsid9021261
71. Chebi:27568
72. Hsdb 6909
73. Selenium Nanopowder In Aq. Media
74. Selenium Metallicum [hpus]
75. Selenium, Elemental [hsdb]
76. Mfcd00011224
77. Db11135
78. Nsc-605850
79. Nsc-605851
80. Nsc-605852
81. N725
82. Q876
83. Selenium, Pellets, < 5mm, >=99.999%
84. E1457
85. Ft-0701285
86. Selenium Ingot/button, ~38mm (1.5in) Dia
87. Selenium And Selenium Compounds [iarc]
88. C01529
89. Selenium Powder [un2658] [keep Away From Food]
90. Selenium Shot, Amorphous, 1-5mm (0.04-0.20in)
91. Selenium, Foil, 25x25mm, Thickness 3mm, 99.95%
92. Hydrogen Selenide, Anhydrous [un2202] [poison Gas]
93. S008000000
94. Selenium, Plasma Standard Solution, Specpure, Se 10g/ml
95. Selenium, Shots, 99.99999% (7n), Diameter 2-4 Mm
96. Selenium, Aas Standard Solution, Specpure?, Se 1000?g/ml
97. Selenium, Pellets, <5 Mm, >=99.99% Trace Metals Basis
98. Selenium, Powder, -100 Mesh, >=99.5% Trace Metals Basis
99. Selenium, Powder, -100 Mesh, 99.99% Trace Metals Basis
100. Selenium, Plasma Standard Solution, Specpure?, Se 1000?g/ml
101. Selenium, Oil Based Standard Solution, Specpure?, Se 1000?g/g
102. Selenium, Plasma Standard Solution, Specpure?, Se 10,000?g/ml
103. Selenium Compound, N.o.s. [un3283] [poison, Keep Away From Food]
104. Selenium, Lump, 3 Mm Max. Lump Size, Weight 100 G, High Purity 99.999%
105. Selenium, Lump, 3 Mm Max. Lump Size, Weight 20 G, High Purity 99.999%
106. Selenium, Lump, 3 Mm Max. Lump Size, Weight 50 G, High Purity 99.999%
107. Selenium, Pellets, <5 Mm Particle Size, >=99.999% Trace Metals Basis
108. Selenium Shot, Amorphous, 2-6mm (0.08-0.2in), Puratronic(r), 99.999% (metals Basis)
109. Selenium, Powder, 250 Max. Part. Size (micron), Purity 99.95%, Weight 100 G
110. Selenium, Powder, 250 Max. Part. Size (micron), Weight 50 G, Purity 99.95%
111. Selenium, Powder, Max. Particle Size 250 Micron, Weight 200 G, Purity 99.95%
112. Selenium, Rod, 99.9999% (6n), Diameter 20 Mm, Length 55 Mm, Approx. 74g
113. Selenium, Rod, 99.99999% (7n), Diameter 20 Mm, Length 55 Mm, Approx. 74 G
114. Se0
115. Selenium Atomic Spectroscopy Standard Concentrate 1.00 G Se, 1.00 G/l, For 1l Standard Solution, Analytical Standard
| Molecular Weight | 78.97 g/mol |
|---|---|
| Molecular Formula | Se |
| Hydrogen Bond Donor Count | 0 |
| Hydrogen Bond Acceptor Count | 0 |
| Rotatable Bond Count | 0 |
| Exact Mass | 79.91652 g/mol |
| Monoisotopic Mass | 79.91652 g/mol |
| Topological Polar Surface Area | 0 Ų |
| Heavy Atom Count | 1 |
| Formal Charge | 0 |
| Complexity | 0 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
/Experimental Therapy/ ... Se-methylselenocysteine (SeMSC), a naturally occurring organic Se product, is considered as one of the most effective chemopreventive selenocompounds. ... Compared with SeMSC, elemental Se at nano size (Nano-Se) possessed equal efficacy in increasing the activities of glutathione peroxidase, thioredoxin reductase, and glutathione S-transferase, but had much lower toxicity as indicated by median lethal dose, acute liver injury, survival rate, and short-term toxicity. /These/ results suggest that Nano-Se can serve as a potential chemopreventive agent with reduced risk of Se toxicity.
PMID:17728283 Zhang J et al; Toxicol Sci 101 (1): 22-31 (2008)
For the supplementation of total parenteral nutrition to prevent hyposelenemia.
FDA Label
Selenium is incorporated into many different selenoproteins which serve various functions throughout the body.
D - Dermatologicals
D11 - Other dermatological preparations
D11A - Other dermatological preparations
D11AC - Medicated shampoos
D11AC03 - Selenium compounds
Absorption
Oral bioavailability of 90% when given as L-selenomethionine. Tmax of 9.17h.
Route of Elimination
Mainly excreted in urine as 1beta-methylseleno-N-acetyl-d-galactosamine and trimethylselenonium. The amount excreted as 1beta-methylseleno-N-acetyl-d-galactosamine plateaus at doses around 2microg after which the amount excreted as trimethylselenonium increases. Some selenium is also excreted in feces when given orally.
Elemental selenium .. is poorly absorbed.
Norderg, G.F. et al; Handbook on the Toxicology of Metals 3rd ed. Academic Press, Burlington, MA. 2007, p. 789
... The distribution and retention of inhaled selenious acid and selenium metal aerosols ... similar in size and chemical form to selenium aerosols that may be produced during fossil fuel combustion /were examined/. Beagle dogs were given 10 to 61 ug Se/kg of body weight by inhalation. Aerosols generated for the inhalation exposures were also collected and instilled into the upper respiratory tracts or stomachs of additional dogs to measure systemic absorption at these sites. Selenium-75, incorporated into the aerosols, was used to determine the Se content in the whole animal, excreta, and individual tissues as a function of time. Virtually all inhaled selenious acid aerosol was rapidly absorbed into the blood from the lungs, gastrointestinal tract, and the nasal membranes. Selenium metal aerosols were less rapidly absorbed. Selenium that was absorbed into the blood was translocated to the liver, kidney, spleen, and heart. Selenium-75 in these organs had a biological half-life of 30 to 40 days. Approximately 50% of the deposited Se was eliminated with a biological half life of 1.2 days. Urine was the major route of excretion, accounting for 70 to 80% of the excreted Se. The long-term component of the whole-body retention function for both inhaled aerosols had a half-life of about 34 days and accounted for about 20% of the initial Se dose. ... Although absorption of selenious acid into blood following inhalation was more rapid than absorption of selenium metal, once absorbed the disposition of both compounds was similar. 6845362
PMID:6845362 Weissman SH et al; Toxicol Appl Pharmacol 67 (3): 331-7 (1983)
Selenium supplements are typically available in the form of sodium selenite which is metabolized to selenide through either glutathione conjugation and subsequent reduction by glutathione reductase enzymes or reduction by thioredoxin reductases. Selenide is further metabolized to selenocystein by cysteine synthases and to selenophosphate by selenophosphate synthases. Selenide is also metabolized progressively to methyl-selenol, dimethyl selenide, then trimethylselenonium. Selenocysteine is degraded to methyl-selenol, pyruvate and ammonia by cysteine beta lyase. Selenocystein reacts with oxygen to form selenocysteine selenoxide which spontaneously degrades to methylselenic acid, pyruvate and ammonia. Methylselenic acid can be converted to methylselenol via conjugation with thiol groups on proteins like glutathione.
Selenium compounds are in part reduced in the body to elemental selenium... Much is metabolized to dimethyl selenide which is excreted by the lungs, imparting a garlic-like odor.
Thienes, C., and T.J. Haley. Clinical Toxicology. 5th ed. Philadelphia: Lea and Febiger, 1972., p. 209
Half life was observed to increase with chronic dosing time. For day 1-2 half life was 1.7 days. For day 2-3 half life was 3 days. For day 3-14 half life was 11.1 days.
Whole body: 140 days; [TDR, p. 1075]
TDR - Ryan RP, Terry CE, Leffingwell SS (eds). Toxicology Desk Reference: The Toxic Exposure and Medical Monitoring Index, 5th Ed. Washington DC: Taylor & Francis, 1999., p. 1075
The distribution and retention of inhaled selenious acid and selenium metal aerosols which were similar in size and chemical form to selenium aerosols that may be produced during fossil fuel combustion /was studied/. Beagle dogs were given 10 to 61 ug Se/kg of body weight by inhalation. ... Approximately 50% of the deposited Se was eliminated with a biological half life of 1.2 days. ... The long-term component of the whole-body retention function for both inhaled aerosols had a half-life of about 34 days and accounted for about 20% of the initial Se dose.
PMID:6845362 Weissman SH et al; Toxicol Appl Pharmacol 67 (3): 331-7 (1983)
Selenium is first metabolized to selenophosphate and selenocysteine. Selenium incorporation is genetically encoded through the RNA sequence UGA. This sequence is recognized by RNA ste loop structures called selenocysteine inserting sequences (SECIS). These structures require the binding of SECIS binding proteins (SBP-2) to recognize selenocystiene. The specialized tRNA is first bound to a serine residue which is then enzymatically processed to a selylcysteyl-tRNA by selenocystiene sythase using selenophosphate as a selenium donor. Other unidentified proteins are required as part of the binding of this tRNA to the ribosome. Selenoproteins appear to be necessary for life as mice with the specialized tRNA gene knocked out exhibited early embryonic lethality. The most important selenoproteins seem to be the glutathione peroxidases and thioredoxin reductases which are part of the body's defenses againts reactive oxygen species (ROS). The importance of selenium in these anti-oxidant proteins has been implicated in the reduction of atherosclerosis by preventing the oxidation of low density lipoprotein. Selenium supplementation is also being investigated in the prevention of cancer and has been suggested to be beneficial to immune function.

API/FDF Prices: Book a Demo to explore the features and consider upgrading later
API Imports and Exports
| Importing Country | Total Quantity (KGS) |
Average Price (USD/KGS) |
Number of Transactions |
|---|
Upgrade, download data, analyse, strategize, subscribe with us
Related Excipient Companies

Excipients by Applications
Market Place

ABOUT THIS PAGE
70
PharmaCompass offers a list of Selenium API manufacturers, exporters & distributors, which can be sorted by GMP, USDMF, JDMF, KDMF, CEP (COS), WC, Price,and more, enabling you to easily find the right Selenium manufacturer or Selenium supplier for your needs.
Send us enquiries for free, and we will assist you in establishing a direct connection with your preferred Selenium manufacturer or Selenium supplier.
PharmaCompass also assists you with knowing the Selenium API Price utilized in the formulation of products. Selenium API Price is not always fixed or binding as the Selenium Price is obtained through a variety of data sources. The Selenium Price can also vary due to multiple factors, including market conditions, regulatory modifications, or negotiated pricing deals.
A Selenium manufacturer is defined as any person or entity involved in the manufacture, preparation, processing, compounding or propagation of Selenium, including repackagers and relabelers. The FDA regulates Selenium manufacturers to ensure that their products comply with relevant laws and regulations and are safe and effective to use. Selenium API Manufacturers are required to adhere to Good Manufacturing Practices (GMP) to ensure that their products are consistently manufactured to meet established quality criteria.
click here to find a list of Selenium manufacturers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PhamaCompass.
A Selenium supplier is an individual or a company that provides Selenium active pharmaceutical ingredient (API) or Selenium finished formulations upon request. The Selenium suppliers may include Selenium API manufacturers, exporters, distributors and traders.
click here to find a list of Selenium suppliers with USDMF, JDMF, KDMF, CEP, GMP, COA and API Price related information on PharmaCompass.
A Selenium DMF (Drug Master File) is a document detailing the whole manufacturing process of Selenium active pharmaceutical ingredient (API) in detail. Different forms of Selenium DMFs exist exist since differing nations have different regulations, such as Selenium USDMF, ASMF (EDMF), JDMF, CDMF, etc.
A Selenium DMF submitted to regulatory agencies in the US is known as a USDMF. Selenium USDMF includes data on Selenium's chemical properties, information on the facilities and procedures used, and details about packaging and storage. The Selenium USDMF is kept confidential to protect the manufacturer’s intellectual property.
click here to find a list of Selenium suppliers with USDMF on PharmaCompass.
Selenium Active pharmaceutical ingredient (API) is produced in GMP-certified manufacturing facility.
GMP stands for Good Manufacturing Practices, which is a system used in the pharmaceutical industry to make sure that goods are regularly produced and monitored in accordance with quality standards. The FDA’s current Good Manufacturing Practices requirements are referred to as cGMP or current GMP which indicates that the company follows the most recent GMP specifications. The World Health Organization (WHO) has its own set of GMP guidelines, called the WHO GMP. Different countries can also set their own guidelines for GMP like China (Chinese GMP) or the EU (EU GMP).
PharmaCompass offers a list of Selenium GMP manufacturers, exporters & distributors, which can be sorted by USDMF, JDMF, KDMF, CEP (COS), WC, API price, and more, enabling you to easily find the right Selenium GMP manufacturer or Selenium GMP API supplier for your needs.
A Selenium CoA (Certificate of Analysis) is a formal document that attests to Selenium's compliance with Selenium specifications and serves as a tool for batch-level quality control.
Selenium CoA mostly includes findings from lab analyses of a specific batch. For each Selenium CoA document that a company creates, the USFDA specifies specific requirements, such as supplier information, material identification, transportation data, evidence of conformity and signature data.
Selenium may be tested according to a variety of international standards, such as European Pharmacopoeia (Selenium EP), Selenium JP (Japanese Pharmacopeia) and the US Pharmacopoeia (Selenium USP).

