



API Suppliers

US DMFs Filed
0

CEP/COS Certifications

JDMFs Filed
0
Other Certificates
0
Other Suppliers
0

USA (Orange Book)
0

Europe
0

Canada

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
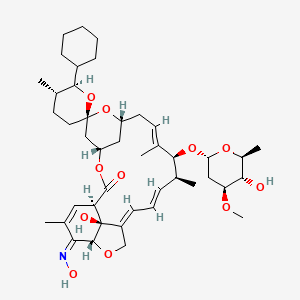

1. 25-cyclohexyl-25-de(1-methylpropyl)-5-deoxy-22 23-dihydro-5-(hydroxyimino)-avermectin B1 Monosaccharide
1. Revolution
2. 220119-17-5
3. Uk-124,114
4. 165108-07-6
5. Uk-124114
6. A2669owx9n
7. Unii-a2669owx9n
8. Stronghold
9. (1r,4s,5's,6r,6's,8r,10e,12s,13s,14e,16e,20r,21z,24s)-6'-cyclohexyl-24-hydroxy-21-hydroxyimino-12-[(2r,4s,5s,6s)-5-hydroxy-4-methoxy-6-methyloxan-2-yl]oxy-5',11,13,22-tetramethylspiro[3,7,19-trioxatetracyclo[15.6.1.14,8.020,24]pentacosa-10,14,16,22-tetraene-6,2'-oxane]-2-one
10. Ncgc00095066-01
11. 25-cyclohexyl-25-de(1-methylpropyl)-5-deoxy-22 23-dihydro-5-(hydroxyimino)-avermectin B1 Monosaccharide
12. Revolution (antibiotic)
13. Selamectin [mi]
14. Selamectin [inn]
15. Selamectin (usan/inn)
16. Selamectin [usan]
17. Selamectin [mart.]
18. Dsstox_cid_25903
19. Dsstox_rid_81215
20. Selamectin [usp-rs]
21. Dsstox_gsid_45903
22. Schembl120105
23. Selamectin [usan:inn:ban]
24. Selamectin [green Book]
25. Chembl1908325
26. Dtxsid6045903
27. Chebi:177562
28. Selamectin [usp Monograph]
29. Ex-a3581
30. Tox21_111408
31. Mfcd31621085
32. Zinc85537134
33. Stronghold Component Selamectin
34. Akos026749796
35. Cs-7778
36. Db11459
37. Nsc 758615
38. Selamectin [ema Epar Veterinary]
39. Selamectin Component Of Stronghold
40. (2ae,4e,5's,6s,6's,7s,8e,11r,13r,15s,17ar,20ar,20bs)-6'-cyclohexyl-7-((2,6-dideoxy-3-o-methyl-alpha-l-arabino-hexopyranosyl)oxy)-3',4',5',6,6',7,10,11,14,15,20a,20b-dodecahydro-20b-hydroxy-5',6,8,19-tetramethylspiro(11,15-methano-2h,13h,17h-furo(4,3,2-pq)(2,6)benzodioxacyclooctadecin-13,2'-(2h)pyran)-17,20(17ah)-dione 20-oxime
41. 25-cyclohexyl-4'-o-de(2,6-dideoxy-3-o-methyl-alpha-l-arabino-hexopyranosyl)-5-demethoxy-25-de(1-methylpropyl)-22,23-dihydro-5-(hydroxyimino)-avermectin A1a
42. Avermectin A1a, 25-cyclohexyl-4'-o-de(2,6-dideoxy-3-o-methyl-.alpha.-l-arabino-hexopyranosyl)-5-demethoxy-25-de(1-methylpropyl)-22,23-dihydro-5-(hydroxyimino)-
43. Selamectin 100 Microg/ml In Acetonitrile
44. Stronghold Plus Component Selamectin
45. Hy-107212
46. Uk 124114
47. Cas-165108-07-6
48. Selamectin Component Of Stronghold Plus
49. D05813
50. J-014423
51. Avermectin A1a, 25-cyclohexyl-4'-o-de(2,6-dideoxy-3-o-methyl-.alpha.-l-arabino-hexopyranosyl)-5-demethoxy-25-de(1-methylpropyl)-22,23-dihydro-5(hydroxyimino)-, (5z)-
52. Avermectin A1a, 25-cyclohexyl-4'-o-de(2,6-dideoxy-3-o-methyl-alpha-l-arabino-hexopyranosyl)-5-demethoxy-25-de(1-methylpropyl)-22,23-dihydro-5-(hydroxyimino)-, (5z)-
| Molecular Weight | 770.0 g/mol |
|---|---|
| Molecular Formula | C43H63NO11 |
| XLogP3 | 5.4 |
| Hydrogen Bond Donor Count | 3 |
| Hydrogen Bond Acceptor Count | 12 |
| Rotatable Bond Count | 4 |
| Exact Mass | 769.44011183 g/mol |
| Monoisotopic Mass | 769.44011183 g/mol |
| Topological Polar Surface Area | 155 Ų |
| Heavy Atom Count | 55 |
| Formal Charge | 0 |
| Complexity | 1550 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 14 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 4 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
- Treatment and prevention of flea infestations caused by Ctenocephalides spp. for one month following a single administration. This is as a result of the adulticidal, larvicidal and ovicidal properties of the product. The product is ovicidal for 3 weeks after administration. Through a reduction in the flea population, monthly treatment of pregnant and lactating animals will also aid in the prevention of flea infestations in the litter up to seven week of age. The product can be used as part of a treatment strategy for flea allergy dermatitis and through its ovicidal and larvicidal action may aid in the control of existing environmental flea infestations in area to which the animal has access.
- Prevention of heartworm disease caused by Dirofilaria immitis with monthly administration. The product may be safely administered to animals infected with adult heartworms, however, it is recommended, in accordance with good veterinary practice, that all animals 6 months of age or more living in countries where a vector exists should be tested for existing adult heartworm infections before beginning medication with the product. It is also recommended that dogs should be tested periodically for adult heartworm infections, as an integral part of a heartworm prevention strategy, even when the product has been administered monthly. This product is not effective against adult D. immitis.
- Treatment of ear mites (Otodectes cynotis).
Cats:
- Treatment of biting lice infestations (Felicola subrostratus)
- Treatment of adult roundworms (Toxocara cati)
- Treatment of adult intestinal hookworms (Ancylostoma tubaeforme).
Dogs:
- Treatment of biting lice infestations (Trichodectes canis)
- Treatment of sarcoptic mange (caused by Sarcoptes scabiei)
- Treatment of adult intestinal roundworms (Toxocara canis).
* Cats and dogs: :
* Treatment and prevention of flea infestations: caused by Ctenocephalides spp. for one month following a single administration. This is as a result of the adulticidal, larvicidal and ovicidal properties of the product. The product is ovicidal for 3 weeks after administration. Through a reduction in the flea population, monthly treatment of pregnant and lactating animals will also aid in the prevention of flea infestations in the litter up to seven weeks of age. The product can be used as part of a treatment strategy for flea allergy dermatitis and through its ovicidal and larvicidal action may aid in the control of existing environmental flea infestations in areas to which the animal has access.
* Prevention of heartworm disease: caused by Dirofilaria immitis with monthly administration.
The product may be safely administered to animals infected with adult heartworms, however, it is recommended, in accordance with good veterinary practice, that all animals 6 months of age or more living in countries where a vector exists should be tested for existing adult heartworm infections before beginning medication with the product. It is also recommended that dogs should be tested periodically for adult heartworm infections, as an integral part of a heartworm prevention strategy, even when the product has been administered monthly. This product is not effective against adult D. immitis.
* Treatment of ear mites: (Otodectes cynotis).
* Cats: :
- Treatment of biting lice infestations (Felicola subrostratus)
- Treatment of adult roundworms (Toxocara cati)
- Treatment of adult intestinal hookworms (Ancylostoma tubaeforme)
- Treatment of biting lice infestations (Trichodectes canis)
- Treatment of sarcoptic mange (caused by Sarcoptes scabiei)
* Cats and dogs: :
-
* Treatment and prevention of flea infestations: caused by Ctenocephalides spp. for one month following a single administration. This is as a result of the adulticidal, larvicidal and ovicidal properties of the product. The product is ovicidal for 3 weeks after administration. Through a reduction in the flea population, monthly treatment of pregnant and lactating animals will also aid in the prevention of flea infestations in the litter up to seven weeks of age. The product can be used as part of a treatment strategy for flea allergy dermatitis and through its ovicidal and larvicidal action may aid in the control of existing environmental flea infestations in areas to which the animal has access.
-
* Prevention of heartworm disease: caused by Dirofilaria immitis with monthly administration. Stronghold may be safely administered to animals infected with adult heartworms, however, it is recommended, in accordance with good veterinary practice, that all animals 6 months of age or more living in countries where a vector exists should be tested for existing adult heartworm infections before beginning medication with Stronghold. It is also recommended that dogs should be tested periodically for adult heartworm infections, as an integral part of a heartworm prevention strategy, even when Stronghold has been administered monthly. This product is not effective against adult D. immitis.
-
* Treatment of ear mites: (Otodectes cynotis).
*:
* Cats: :
- Treatment of biting lice infestations (Felicola subrostratus
- Treatment of adult roundworms (Toxocaracati)
- Treatment of adult intestinal hookworms (Ancylostoma tubaeforme).
*:
* Dogs: :
- Treatment of biting lice infestations (Trichodectes canis)
- Treatment of sarcoptic mange (caused by Sarcoptes scabiei)
- Treatment of adult intestinal roundworms (Toxocara canis).
Antiparasitic Agents
Drugs used to treat or prevent parasitic infections. (See all compounds classified as Antiparasitic Agents.)
QP54AA05
QP54AA05
QP54AA05


