


API Suppliers
0

US DMFs Filed
0

CEP/COS Certifications
0

JDMFs Filed
0
EU WC
0
Listed Suppliers
0
0

USA (Orange Book)
0

Europe
0

Canada
0

Australia
0

South Africa
0
Uploaded Dossiers
0
U.S. Medicaid
0
Annual Reports
0
0
USFDA Orange Book Patents
0
USFDA Exclusivities
0
Blog #PharmaFlow
0
News
0
EDQM
0
USP
0
JP
0
Other Listed Suppliers
0
0
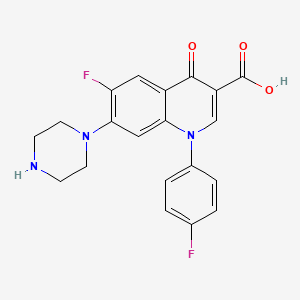

1. 1-(4-fluorophenyl)-6-fluoro-7-(1-piperazinyl)-1,4-dihydro-4-oxo-quinoline-3-carboxylic Acid
2. 1-fpfpoc
3. 6-fluoro-1-(4-fluorophenyl)-7-piperazinyl-1,4-dihydro-4-quinolone-3-carboxylic Acid
4. A 56620
5. A-56620
6. Sarafloxacin Hydrochloride
1. 98105-99-8
2. Sarafloxacine
3. 6-fluoro-1-(4-fluorophenyl)-4-oxo-7-(piperazin-1-yl)-1,4-dihydroquinoline-3-carboxylic Acid
4. Difloxacino
5. Difloxacinum
6. Sarafloxacino
7. Sarafloxacinum
8. Sarafloxacin [inn:ban]
9. Quinolone Der.
10. 3-quinolinecarboxylic Acid, 6-fluoro-1-(4-fluorophenyl)-1,4-dihydro-4-oxo-7-(1-piperazinyl)-
11. Rc3wj907xy
12. Abbott-57135
13. Saraflox
14. Sarafloxacin (inn)
15. Sarafloxacin [inn]
16. 6-fluoro-1-(4-fluorophenyl)-4-oxo-7-piperazin-1-ylquinoline-3-carboxylic Acid
17. Difloxacine [french]
18. Difloxacinum [latin]
19. Difloxacino [spanish]
20. Abbott 57135
21. 6-fluoro-1-(4-fluorophenyl)-4-oxo-7-piperazin-1-yl-quinoline-3-carboxylic Acid
22. Sarafloxacine [inn-french]
23. Sarafloxacinum [inn-latin]
24. Sarafloxacino [inn-spanish]
25. Sarafloxacin Hydrochloride Trihydrate
26. Hsdb 7035
27. Unii-rc3wj907xy
28. 6-fluoro-1-(4-fluorophenyl)-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic Acid
29. Ccris 8500
30. Sr-05000002002
31. Spectrum2_000036
32. Spectrum3_001953
33. Sarafloxacin [mi]
34. Sarafloxacin [hsdb]
35. Dsstox_cid_28468
36. Dsstox_gsid_48494
37. Bspbio_003553
38. Mls006011798
39. Chembl37858
40. Schembl311593
41. Spbio_000131
42. Dtxsid8048494
43. Chebi:94493
44. Gtpl10857
45. Kbio3_002868
46. Hms2090n09
47. Hms3715k18
48. Zinc538330
49. Albb-028536
50. Rkl10086
51. Tox21_303838
52. Bbl010397
53. Mfcd00865974
54. Stk713346
55. Akos004938890
56. Ccg-221098
57. Db11491
58. Ks-5005
59. Ncgc00177995-01
60. Ncgc00177995-03
61. Ncgc00177995-04
62. Ncgc00356946-01
63. Ac-12861
64. Pd121960
65. Smr001550490
66. Sbi-0206753.p001
67. Cas-98105-99-8
68. Db-057710
69. Pd 121960
70. Cs-0013790
71. Ft-0630998
72. A56620
73. D08506
74. E73929
75. Ab00923780-04
76. Ab00923780_06
77. Ab00923780_07
78. 105s998
79. A 57135
80. A845810
81. A858584
82. Q7421984
83. Sr-05000002002-2
84. Brd-k08525451-003-03-7
85. Sarafloxacin, Sarafloxacin Hydrochloride, Sarafloxacin Hcl
86. 1-p-fluoro-phenyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)quinoline-3-carboxylic Acid
87. 6-fluoranyl-1-(4-fluorophenyl)-4-oxidanylidene-7-piperazin-1-yl-quinoline-3-carboxylic Acid
88. 6-fluoro-1-(4-fluorophenyl)-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic Acid
89. 6-fluoro-1-(4-fluorophenyl)-4-oxo-7-piperazin-1-yl-1,4-dihydroquinoline-3-carboxylic Acid
90. 3-quinolinecarboxylic Acid, 6-fluoro-1-(4-fluorophenyl)-1,4-dihydro-4-oxo-7-(1-piperazinyl)-, Hydrochloride
91. 6-fluoro-1-(4-fluorophenyl)-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic Acid, 9ci
92. 6-fluoro-1-(4-fluorophenyl)-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic Acid;sarafloxacin
93. 6-fluoro-1-(p-fluorophenyl)-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinolinecarboxylic Acid
| Molecular Weight | 385.4 g/mol |
|---|---|
| Molecular Formula | C20H17F2N3O3 |
| XLogP3 | 0.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 8 |
| Rotatable Bond Count | 3 |
| Exact Mass | 385.12379774 g/mol |
| Monoisotopic Mass | 385.12379774 g/mol |
| Topological Polar Surface Area | 72.9 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 645 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently Bonded Unit Count | 1 |
MEDICATION (VET): /Sarafloxacin/ is used for treatment and control of bacterial infections in poultry caused by Escherichia coli and Salmonella spp.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 41: Sarafloxacin (1998). Available from, as of October 7, 2015: https://www.inchem.org/pages/jecfa.html
MEDICATION (VET): Infections of chickens with Escherichia coli serotype O78 can be treated with the antibiotic sarafloxacin. Three experiments were conducted on the administration of this drug to chickens that had been experimentally infected with E. coli. The birds were monitored for 10 days after infection for their average daily gain (ADG) and feed conversion ratio (FCR), and the post-mortem pathology was assessed. In the first experiment, sarafloxacin (20 mg/L, equivalent to 5 mg/kg live weight per day), given in the drinking water for 3 days after infection, led to a reduction in the mortality from 75% to 27%, but the ADG of the treated birds was still less than that of the uninfected controls. In the second experiment, when the sarafloxacin was administered at the same dose in the water but over only 2 hr, there was also a considerable reduction in mortality, and the ADG and the FCR also improved significantly. In the third experiment, the dose dependence of the drug was tested. The birds were given 5 and 10 mg/kg per day sarafloxacin in each group, starting within 2 hr after infection. This rapid administration of the drug completely prevented mortality, while the ADG and FCR were similar to those of the uninfected controls.
PMID:12184496 Chansiripornchai N, Sasipreeyajan J; Vet Res Commun 26 (4): 255-62 (2002)
MEDICATION (VET): Antibiotics authorized for use in aquaculture: Sarafloxacin - Indicated in the treatment of furunculosis, vibriosis and enteric redmouth in Salmonidae. /From table/
WHO/FAO; FAO Fisheries Technical Paper 469: Responsible Use of Antibiotics in Aquaculture, p9 (2005). Available from, as of October 8, 2015: https://www.fao.org/3/a-a0282e.pdf
(14)C-Sarafloxacin was orally administered to six laying hens for five consecutive days. Eggs were collected for 15 days after the initial drug treatment. Egg yolk and egg albumen were separated and assayed for total radioactive residues (TRR) using a combustion oxidizer and scintillation counting techniques. Radioactivity was detected in egg yolk and egg albumen on the second day of dosing and reached a maximum at 24 hr after drug withdrawal. Thereafter, the sarafloxacin TRR levels in egg albumen declined rapidly and were undetectable 2 days after the last dose, whereas the levels in egg yolk declined at a much slower rate and were undetectable 7 days after drug withdrawal. In both the egg albumen and yolk, HPLC analysis indicated that the parent sarafloxacin was the major component.
PMID:11141293 Chu PS et al; J Agric Food Chem 48 (12): 6409-11 (2000)
Pharmacokinetics of sarafloxacin, a fluoroquinolone antibiotic, was determined in pigs and broilers after intravenous (i.v.), intramuscular (i.m.), or oral (p.o.) administration at a single dose of 5 (pigs) or 10 mg/kg (broilers). Plasma concentration profiles were analysed by a noncompartmental pharmacokinetic method. Following i.v., i.m. and p.o. doses, the elimination half-lives were 3.37 +/- 0.46, 4.66 +/- 1.34, 7.20 +/- 1.92 (pigs) and 2.53 +/- 0.82, 6.81 +/- 2.04, 3.89 +/- 1.19 hR (broilers), respectively. After i.m. and p.o. doses, bioavailabilities (F) were 81.8 +/- 9.8 and 42.6 +/- 8.2% (pigs) and 72.1 +/- 8.1 and 59.6 +/- 13.8% (broilers), respectively. Steady-state distribution volumes (Vd(ss)) of 1.92 +/- 0.27 and 3.40 +/- 1.26 L/kg and total body clearances (ClB) of 0.51 +/- 0.03 and 1.20 +/- 0.20 L/kg/hr were determined in pigs and broilers, respectively. Areas under the curve (AUC), mean residence times (MRT), and mean absorption times (MAT) were also determined. Sarafloxacin was demonstrated to be more rapidly absorbed, more extensively distributed, and more quickly eliminated in broilers than in pigs. Based on the single-dose pharmacokinetic parameters determined, multiple dosage regimens were recommended as: a dosage of 10 mg/kg given intramuscularly every 12 hr in pigs, or administered orally every 8 hr in broilers, can maintain effective plasma concentrations with bacteria infections, in which MIC90 are <0.25 ug/mL.
PMID:11696079 Ding HZ et al; J Vet Pharmacol Ther 24 (5): 303-8 (2001)
The absorption, metabolism, and excretion of (14)C-labelled sarafloxacin was studied in three-month-old female New Zealand white rabbits. Two groups of three animals per group were treated orally by gavage with 10 mg/kg bw of (14)C-sarafloxacin base. A third group of three animals received the same dose by intravenous administration. Blood samples were collected 1, 3, 6, 12, and 24 hr after oral administration from animals in one of the groups, and urine and feces were collected daily for five days from animals in the other groups. ... Within five days of oral administration, about 11% of the dose was eliminated in the urine and about 79% in the feces. Urinary excretion after intravenous administration indicated that about 16% of the oral dose had been systemically absorbed.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 41: Sarafloxacin (1998). Available from, as of October 7, 2015: https://www.inchem.org/pages/jecfa.html
Five groups of 18 Sprague-Dawley rats of each sex were treated with sarafloxacin as follows: One group received a single intravenous dose of 20 mg/kg bw; three groups received a single oral dose of 20, 75, or 275 mg/kg bw; and animals in the fifth group received an oral dose of 1000 mg/kg bw daily for 14 consecutive days. Blood samples were collected from four rats in each group just before treatment and 0.5, 1, 2, 4, 6, 8, 12, and 24 hr after treatment on day 1 for the groups receiving the single dose and on days 1 and 14 for the 14-day treatment group. Plasma and urine samples were assayed for sarafloxacin base by high-performance liquid chromatography. ... A comparison of the 0 to infinity area under the concentration time curve (AUC) after a single intravenous or oral dose of 20 mg/kg bw sarafloxacin indicated that its bioavailability was about 12%. A plot of the AUC against dose was linear up to 275 mg/kg bw but deviated from linearity at 1000 mg/kg bw.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 41: Sarafloxacin (1998). Available from, as of October 7, 2015: https://www.inchem.org/pages/jecfa.html
For more Absorption, Distribution and Excretion (Complete) data for SARAFLOXACIN (8 total), please visit the HSDB record page.
The pharmacokinetics and metabolism of sarafloxacin were studied in two groups of six volunteers given a single oral dose of 100 or 200 mg sarafloxacin and two groups of five volunteers given a single oral dose of 400 or 800 mg. ... The metabolism of sarafloxacin appears to involve mainly oxidative degradation of the piperazinyl substituent, first producing 3'-oxo-sarafloxacin. Subsequent oxidation produces an ethylene diamine-substituted quinolone, which in turn is oxidized to an aminoquinolone. The plasma concentrations of the ethylene diamine-substituted quinolone parallel those of the parent drug, but the average AUC for the quinolone was consistently only about 6% that of sarafloxacin. The concentration of the aminoquinolone in plasma and urine was considerably lower than that of the ethylene diamine-substituted quinolone. Owing to its weak fluorescence, 3'-oxo-sarafloxacin was not detected in plasma. In urine, the major drug-related peak was sarafloxacin, accounting for 75-80% of all urinary metabolites. After sarafloxacin, the predominant metabolite in urine was tentatively identified as 3'-oxo-sarafloxacin, which occurred at concentrations that were typically one-third to one-fourth those of sarafloxacin. The total urinary recovery of parent drug plus metabolites was low and dose-dependent, decreasing from 24 to 10% as the dose increased from 100 to 800 mg. The extent of the decrease was similar to that in the dose-normalized AUC. Collectively, the aminoquinolone, the ethylene diamine-substituted quinolone, and their conjugates accounted for < 7% of the urinary excretion.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 41: Sarafloxacin (1998). Available from, as of October 7, 2015: https://www.inchem.org/pages/jecfa.html
... /Dogs (breed, sex, and number not stated) were given an oral or intravenous dose of 10 mg/kg bw dose of (14)C-sarafloxacin base./ ... About 79% of the 10 mg/kg bw dose of (14)C-sarafloxacin base was excreted as unmetabolized parent drug in urine and faeces. In bile, the unchanged parent drug and its glucuronide were found in about equal proportions
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 41: Sarafloxacin (1998). Available from, as of October 7, 2015: https://www.inchem.org/pages/jecfa.html
To investigate the microbial biotransformation of veterinary fluoroquinolones, Mucor ramannianus was grown in sucrose/peptone broth with sarafloxacin for 18 days. Cultures were extracted with ethyl acetate and extracts were analyzed by liquid chromatography. The two metabolites (26% and 15% of the A280, respectively) were identified by mass and 1H nuclear magnetic resonance spectra as N-acetylsarafloxacin and desethylene-N-acetylsarafloxacin. The biological formation of desethylene-N-acetylsarafloxacin has not been previously observed.
PMID:11420653 Parshikov IA et al; J Ind Microbiol Biotechnol 26 (3): 140-4 (2001)
A single oral dose of 100, 200, 400, or 800 mg sarafloxacin was administered to 22 healthy male volunteers ranging in age from 20 to 39 years. ... The average terminal phase half-lives were 9, 9, 10, and 11 hr at the 100, 200, 400, and 800 mg doses, respectively.
WHO/FAO; Joint Meeting on Food Additives; Toxicological Evaluation of Certain Veterinary Drug Residues in Food, WHO Food Additive Series 41: Sarafloxacin (1998). Available from, as of October 7, 2015: https://www.inchem.org/pages/jecfa.html
Pharmacokinetics of sarafloxacin, a fluoroquinolone antibiotic, was determined in pigs and broilers after intravenous (i.v.), intramuscular (i.m.), or oral (p.o.) administration at a single dose of 5 (pigs) or 10 mg/kg (broilers). ... Following i.v., i.m. and p.o. doses, the elimination half-lives were 3.37 +/- 0.46, 4.66 +/- 1.34, 7.20 +/- 1.92 (pigs) and 2.53 +/- 0.82, 6.81 +/- 2.04, 3.89 +/- 1.19 hR (broilers), respectively. ...
PMID:11696079 Ding HZ et al; J Vet Pharmacol Ther 24 (5): 303-8 (2001)
The pharmacokinetics of sarafloxacin applied by oral gavage at a dose of 15 mg/kg bw was studied in eel (Anguilla anguilla) at water temperature of 24 degrees C. ... The distribution rate constant (alpha) was 0.085 hr(-1) (r=0.972), and the half-life (t(1,2alpha)) was 8.15 hr. The elimination rate constant (beta) was 0.023 hr(-1) (r=0.909), and the half-life (t(1/2beta)) was 30.13 hr. ...
PMID:10379934 Ho SP et al; J Vet Med Sci 61 (5): 459-63 (1999)


